The emerging novel coronavirus SARS-CoV-2 and the corresponding disease COVID-19 is undoubtedly one of the most discussed public health issues at the moment. The primary manifestation of COVID-19 is in most cases that of a mild respiratory infectious disease. Still, COVID-19 has the potential to progress to severe viral pneumonia, respiratory failure and a catastrophic cytokine-release-like systematic inflammatory syndrome [1]. After the diagnosis and initial management of COVID-19 patients, the treating physicians must shift their attention to the severe complications accompanying the disease. Among these, and perhaps one of the less reported in literature, is the occurrence of secondary spontaneous pneumothorax (SSP) as a result of severe lung tissue damage.
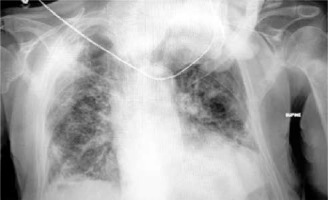
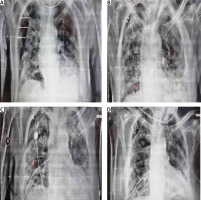
On 24/8/2021, a 78-year-old man presented at the Emergency Department of our institution, complaining of sudden severe dyspnea, and reporting malaise as well as dry cough for the past 3 days. The patient’s past medical history included diabetes mellitus and primary hypertension, for which he was treated with metformin and lisinopril. The patient was a past smoker who had quit smoking for the past 7 years. The first vaccination dose of the Pfizer–BioNTech COVID-19 vaccine was also administered to the patient, only a few days prior, on 19/08/2021. On physical examination, the patient appeared distressed, dyspneic and tachypneic. Respiratory rate was 30 breaths/min with O2 saturation of 89% on room air and febrile with a temperature of 38.5°C. The rest of the vital signs were found to have normal values: heart rate of 80 bpm, blood pressure of 125/73 mm Hg. Examination of the respiratory system revealed diffuse rales at the lower and middle pulmonary fields on auscultation. The rest of the physical examination was unremarkable. Laboratory examinations of the patient included a rapid antigen test for SARS-CoV-2 infection, due to the classification of the patient’s presentation as probable COVID-19 pneumonia, in accordance with the current national guidelines. The nasopharyngeal swab of the patient tested positive, and he was subsequently placed within the high-dependency unit (HDU), on high-flow nasal cannula (HFNC) oxygen therapy, and closely monitored for further deterioration that would require immediate invasive respiratory support. Arterial blood gas testing revealed a partial O2 pressure of 58.9 mm Hg, indicating impending respiratory failure. Laboratory tests also revealed CRP levels of 2.72 mg/dl (ref. < 1.5 mg/dl) on admission, blood glucose levels of 126 mg/dl (ref. 70–115 mg/dl) and WBC count of 16.7 × 109/l. The patient remained stable, and on high-flow oxygen therapy inside the HDU. On 27/8/2021 the patient was started on a course of methylprednisolone in accordance with current guidelines on the management of hospitalized COVID-19 patients. The addition of azithromycin was also deemed appropriate, due to high suspicion of bacterial superinfection. The patient was hospitalized for 26 days, gradually showing signs of improved pulmonary function, until 19/9/2021, when he reported a sudden, acute, new dyspnea exacerbation. Physical examination revealed diminished respiratory sounds on the right lung. Emergency chest X-ray was requested and revealed the presence of pneumothorax on the right lung (Figure 1 A). An emergency chest drainage tube was placed under local anesthesia. Radiological examination of the patient after the chest tube placement revealed partial lung re-expansion (Figure 1 B). Air drainage remained continuous over the next days, indicating persistent air leak and incomplete lung re-expansion. On attempting to stop the suction of the drainage, the pneumothorax grew further, meaning a continuous underlying air loss. Due to the failure to resolve the pneumothorax, definitive surgical management of the patient was elected by the treating physicians. On the 32nd post-admission day the patient underwent an attempted VATS pleurodesis. Under direct visual examination, the surface of the lung was found to be severely fibrotic on appearance, which explained the inability to achieve full re-expansion. The site of air leak was localized at the apex, which also did not achieve re-expansion and contact with the pleural wall. The underlying lung fibrosis greatly restricted the ability to utilize staplers, and the surgical team decided to use an intraoperative fibrinogen/thrombin surgical patch that was held in place with simple interrupted sutures. After completion of the procedure, the patient was successfully extubated, and was returned to the HDU for further monitoring. The postoperative radiological assessment revealed once again partial re-expansion of the affected fibrotic lung (Figure 1 C). Clinically, the patient had the highest O2 dependency since admission, and monitoring of the thoracic drainage tube indicated successful treatment of the continuous air loss. Nine days later, on the 41st post-admission day, the patient once again complained of sudden, severe dyspnea, and the drainage tube revealed recurrence of the air leak at the pre-surgery levels, nonetheless. The multidisciplinary team of physicians decided to make use of intrapleural instillation of fresh frozen plasma (FFP) via the drainage tube. The procedure required 3 units of FFP, and the drainage system was modified, with the placement of the tube above chest level and under continuous suction, in order to drain the remaining air but not the FFP fluid. The air leak once again stopped, and a partial lung re-expansion was again noted on a new chest X-ray (Figure 1 D). After 10 days, the patient remained stable, reported no new dyspnea and the air leak had completely subsided. The chest drainage tube was removed, and the patient was discharged home. The final radiological image prior to discharge can be seen in Figure 2.
Figure 1
White arrows indicate initial pneumothorax size (A), partial lung re-expansion after initial chest tube insertion. White arrows indicate pneumothorax size. The red arrow indicates the chest tube (B), partial lung re-expansion after surgical procedure, and surgical patch placement. The white arrow indicates pneumothorax size. The red arrow indicates the chest tube (C), partial lung re-expansion, without the presence of air leak after 3 units of FFP were instilled. White arrows indicate pneumothorax size. The red arrow indicates the chest tube (D)
Secondary spontaneous pneumothorax is most commonly found in patients with known structural deformities of the lung, patients with cystic fibrosis, other causes of cystic lung abnormalities (e.g. amyloidosis, lymphoid interstitial pneumonia, Sjogren syndrome and more), chronic smokers and COPD patients. More rarely, SSP can occur as a complication of a destructive pulmonary infection. The pathogens most notably associated with SSP are Pneumocystis jirovecii, Mycoplasma pneumoniae and Mycobacterium tuberculosis [2, 3]. With the spread of the novel coronavirus, several centers have reported SSP as a complication of COVID-19 associated pneumonia. A recent meta-analysis of patient registries concluded that pneumothorax has a low incidence of 0.97% of admitted patients, with previous, smaller analyses estimating an incidence of 0.3–0.6% of the hospitalized patients. The incidence of COVID-19 related pneumothorax can however rise to 12.8–23.8% in patients requiring invasive mechanical ventilation [4]. About half of the patients developing COVID-19-related pneumothorax are not on invasive ventilation support. The majority of the patients who develop pneumothorax as a complication of COVID-19 pneumonia require chest tube thoracostomy, as is reported in large retrospective studies [4]. Thoracoscopic surgical management was required in approximately 2% of the studied patients of a recent meta-analysis, with no reports explicitly mentioning two attempts of pleurodesis, making our case unique in this aspect [4]. The reason behind our ineffective first attempt to seal the pneumothorax was that the use of any means of pleurodesis requires lung re-expansion to achieve successful adhesion to the pleura. In contrast, FFP use that does not require full lung re-expansion was successful in stopping the air leak and treating the pneumothorax. Risk factors for the development of COVID-19-related pneumonia include pre-existing lung disease, although more than half of the studied patients in one large study did not report any pre-existing diagnosed conditions, like our patient [4]. Patients who were former smokers, like in our case, had a calculated odds ratio of 1.44 in a multivariate analysis of risk factors for pneumothorax, revealing both active and former smoker status as an independent prognostic factor for the development of COVID-19-related pneumothorax [5]. The prognosis of patients with COVID-19 related pneumothorax seems to be worse for people aged over 70 years and patients who developed acidosis during their hospitalization. Overall mortality rate is currently estimated at high levels, between 74.2% and 88.3% [6]. It is worth noting, however, that studies estimating mortality rates do not report separate mortality rates for patients not on invasive mechanical ventilation support, for whom mortality is expected to be lower. In our case, the patient was deemed a good surgical candidate in order to undergo VATS surgery, since despite his unresolved air leak, his clinical condition had improved, and the supplemental O2 therapy was deescalated to simple nasal-cannula-delivered O2. Despite the improved clinical condition, VATS was quickly deemed impossible, since the lung fibrosis would not allow for stapler use. The utilization of chemical pleurodesis measures was reserved as a last resort in our case for several reasons. First, the effective use of talc and other sclerosants requires the full re-expansion of the lung, in order to maximize the chances of success of the pleurodesis. This was not the case with our patient. Another reason for attempting a surgical pleurodesis first was that the development of fibrous tissue after a chemical pleurodesis would constitute a re-operation much less feasible, and with lower chances of success in an already moderate-to-high risk surgical patient. Upon entry to the pleural cavity, it became apparent that the extensive fibrosis that had developed would not allow for pleurodesis, or bullectomy of the visible point of the air leak. The use of FFP instillation for the successful treatment of recurrent COVID-19 associated pneumothorax is another novel aspect of our herein reported case. Typical choices for chemical pleurodesis include talc, tetracycline derivatives and iodopovidone [5]. More rarely, autologous blood has been utilized for the purposes of pleurodesis, with encouraging results. All of the above require full lung re-expansion, a perquisite not in place for the utilization of FFP. Use of FFP for treating pneumothorax is an alternative, highly successful method of pleurodesis. Despite not being evaluated in large, randomized trials, FFP instillation has proven to be a reliable means of pleurodesis with minimal to no complications being reported from its use [7]. On the other hand, use of chemical pleurodesis compounds has been associated with a variety of rare, yet alarming side effects. Intrapleural injection of talc has been associated with a systematic inflammatory response that can progress to ARDS and respiratory failure [8]. Arrhythmias, myocardial infarction, hypotension and cardiac arrest have also been rarely reported with chemical pleurodesis. Use of autologous blood for pleurodesis was found to rarely cause empyema, as was the use of talc in up to 11% of patients [9]. Reports on FFP utilization show no associated complications, as well as high success rates in treating air leaks. Taking all of the above into consideration, as well as the already high burden of the patient, the surgical team decided upon utilization of readily available FFP units instead. To the best of our knowledge, this is the first report of FFP employed for chemical pleurodesis in a patient with COVID-19 related pneumonia.