Introduction
Since 1990, when first cholecystectomy was made by laparoscopic approach, minimally invasive approaches in surgery have noticed a great development. In 1993, after 33 years from the first aortic valve surgery Rao and Kumar performed first minimal invasive aortic valve surgery [1].
Minimal invasive accesses due to shorter skin incisions and better cosmetic results may be recommended for a group of young patients. Also, minimal invasive access due to less bleeding, reduced ventilation time, reduced stay in the intensive care unit and shorter hospitalization [2] may be an interesting solution in high-risk, elderly patients who are not qualified for transcatheter aortic valve implantation (TAVI) procedure.
According to Aliahmed, minimally invasive approaches reduce the amount of blood loss and probability of infection [3]. The same author also presents few disadvantages of minimally invasive access such as longer cross-clamp and cardiopulmonary bypass times [3].
Several authors reported advantages of ministernotomy access in reoperation. Tabata et al. reported reduced blood transfusion and decreased operative time relative to a full sternotomy [4].
Several minimally invasive accesses are used in cardiac surgery. The most commonly used approaches for aortic valve are upper J hemisternotomy and the right anterior minithoracotomy. There are also known two other accesses by parasternal incision and transverse sternotomy. Both were abandoned because of the risk of lung herniation and disfigurement and were difficult to switch to a full sternotomy if needed [1].
Owing to the longer cross-clamp times and cardiopulmonary bypass times caused by a higher grade of complexity of the procedure, these approaches did not gain wide popularity [5]. The use of sutureless valves like Perceval™ (Corcym, UK) and Intuity™ (Edwards Lifesciences, USA), Enable 3F™ (Medtronic, USA) could give a lot of benefits [6, 7].
This feature, because of shorter cross-clamp and CPB times, gives a benefit for elderly patients with higher Euro-SCORE II [5]. One of the benefits in some sutureless valves is that the valve before implantation is collapsed which enhancing direct visualization [5]. This is a feature which could be helpful for the minimally invasion approach. One of the disadvantages of sutureless valves is price, which is higher than conventional valves, but cheaper than TAVI valves.
Aim
The aim of our study was to assess the results after sutureless valve implantation in comparison to standard valve implantation from upper ministernotomy approach.
Material and methods
Patients
The quasi-experimental retrospective non-randomized study involved 76 consecutive patients (51 males and 25 females) with mean age of 63.2 years who were treated with minimally invasive aortic valve replacement (miniAVR) from upper partial sternotomy between 2015 and 2022. The surgeries were performed exclusively by the most experienced cardiac surgeons with at least 10-year practice in operations on the aortic valves. The miniAVR individuals were divided into 2 subgroups: group I (n = 40; 23 men and 17 women) with sutureless bioprostheses and group II (n = 36; 27 men and 8 women) with mechanical prostheses. The main factor for patient allocation was patient preferences in prostheses chosen – the inclusion criterion for group I was bioprostheses and for group II – mechanical prostheses. The selected demographic variables are outlined in Table I. The STROBE checklist was applied in this study.
Preoperative clinical characteristics
A non-equivalent comparison group, quasi-experimental design was employed to include patients who were qualified for elective surgery on the basis of echocardiographic examination (M + 2D + Doppler). The most common indication was isolated severe aortic stenosis (20 patients in group I and 23 in group II), followed by combined aortic valve disease (17 and 6 cases, respectively), isolated aortic insufficiency (3 and 7 cases). One patient in group I and one in group II were operated due to infective endocarditis. The miniAVR subjects have suffered from many comorbidities. The most common are summarized in Table II. Group I patients were older with lower glomerular filtration rate (GFR) and higher EuroSCORE (p < 0.001; p < 0.001; p = 0.0308).
Table II
Comorbidities for each group (n = 76)
Surgical details
All patients were operated on through partial (upper) ministernotomy (to the 3rd intercostal space) in the cardio-pulmonary bypass (CPB) and with cold cardioplegic crystalloid arrest (Bretschneider formula) infused directly to the coronary ostia. In all but one, CPB was conducted through direct aortic and right atrial cannulation. After either native valve was removed, sutureless or mechanical prostheses were implanted. In standard mechanical and biologic valve implantation all knots were performed manually. The further steps of surgery were typical, and the chest was always closed with 4 sternal wires. Two drains were introduced through the right plural cavity; one of them was left in this pleura and another one in the pericardium around the ascending aorta.
Main variables of interest
From each patient we collected preoperative information, specific data according to the indication for valve surgery, intraoperative data, and postoperative data. Technical and clinical success with patient mortality, survival and reoperation rate were evaluated.
Analyzed clinical complications were infection, postoperative bleeding requiring repeat surgery, postoperative permanent pacemaker implantation, heart arrythmia and cerebrovascular accident (CVA), renal failure with a need for dialysis, respiratory failure, and intestinal ischemia. Each patient was followed up regularly in the Cardiac Surgical Outpatient Clinic. The clinical assessment as well as imaging examinations with transthoracic echocardiography (TTE) were done 1, 6 and 12 months after procedures and then once a year. In mid-term outcomes the assessment of sternum and wound healing, as well as survival from 1.5 to 100.9 follow-up period was performed.
Data management and analysis
The data analysis was performed anonymously. First, the quantitative variables were checked for normality by means of the W Shapiro Wilk test and because they did not satisfy criteria of normal distribution, the Mann Whitney U test and Fisher’s Exact test was used. Data are presented as the median with range (minimum–maximum). Categorical data are expressed as number (n) with percent (%). Probability of survival was estimated by means of the Kaplan-Meier method. The post-discharge follow-up survival probability was compared with the use of the log-rank test. The p-value less than 0.05 was considered statistically significant. All statistical analyses were performed in the Statistica 13.3 software package (TIBCO Software Inc., Palo Alto, CA, USA).
Results
Surgical approach
In all cases partial upper sternotomy up to the 3rd intercostal space was technically successful. The median aorta cross clamping time as well as CPB time were significantly shorter in group I than II (p < 0.001) (Table III).
In-hospital outcomes
The technical success of performed procedures was 100%. One patient in group I and one patient from group II did not survive and died after the surgery due to cardiogenic shock and brain death. Prior to the surgery patients were in stable condition, with comorbidities like diabetes, chronic renal failure, hypertension (patients in group I) and heart failure (patient in group II). Of note, one patient who survived the 30-day postoperative period died due to multi-organ failure (MOF) later (on the 45th day) during hospitalization directly related to the operation. The clinical success defined as survival during the in-hospital period time was 95% in group I and 97.3% in group II.
We did not observe any wound infections during hospitalization. In one patient in group II the reoperation because of bleeding was necessary. Renal failure and dialysis were necessary in 3 patients in group I and one in group II. Respiratory failure with prolonged mechanical ventilation occurred in 1 (2%) patient in group I and 2 patients in group II. We did not observe intestinal ischemia and any cerebrovascular accident (CVA) during hospitalization. Postoperative permanent pacemaker implantation because of AV block after surgery was necessary in 8 (20%) patients in group I and in 3 (8%) cases in group II (p = 0.19). In our opinion, a higher rate of AV block in group I could be caused by balloon dilatation which provides optimal sealing of the valve to the aortic annulus. Half of patients from group I had at least one episode of atrial fibrillation that was successfully treated pharmacologically with intravenous amiodarone. In group II with mechanical valve implantation there were 11 (30%) patients with AF, and 1 (2%) with the nsVT episode. No other supra- and ventricular arrythmias were observed.
In 87.5% of cases, red cell concentrate was transfused in group I. The median (minimum; maximum) transfused blood units were 4 (0; 12) for the group of patients with sutureless valve implantation, versus 94% of cases that needed blood transfused in the group with mechanical valve implantation. Median transfused blood units were 2 (0; 6). 67.5% of patients had fresh frozen plasma transfused. The median fresh frozen plasma unit was 1 (0; 10) versus 72% of cases in the group with mechanical valve implantation, fresh frozen plasma transfusion was needed. The median unit was 1 (2; 0).
In echocardiography performed before discharge, the function of implanted prostheses was correct in all cases. Median EF was 60%. No cases of PVL were noted. All detailed data regarding early post-operative course are presented in Table IV.
Table IV
Post operative course (n = 76)
Long-term follow-up
The follow-up period ranged from 1.5 to 100.9 months and was completed by all subjects who were discharged alive (38 patients (95.0%) in group I, and 35 (97.2%) in group II). Of note, life expectancy was much longer (median (min.; max.)) in group II (63.8 (7.6; 97.6) months) than in group I (26.4 (1.5; 100.9) months) (p < 0.001).
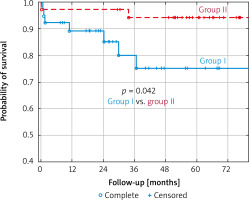
Throughout the post-discharge follow-up period, 5 deaths in group I and only 1 in group II were noted. In consequence, annual, 2-year and 5-year probabilities of survival according to the Kaplan-Meier method were 0.89 ±0.05 vs. 0.97 ±0.03, 0.85 ±0.06 vs. 0.97 ±0.03 and 0.75 ±0.10 vs. 0.94 ±0.04, in group I and II, respectively (p = 0.042).
After discharge from hospital no wound infection or sternum instability was reported.
In the last follow-up transthoracic echocardiography studies, the correct prosthesis position and function were observed in all individuals. There were no cases of graft dislocation, PVL and no signs of structural valve deterioration (SVD) observed. Of note, left ventricular systolic performance recovered completely and EF was found within normal range in all survivors. It corresponded with functional status estimated at the end of the follow-up period. All of them were found in NYHA functional classes I and II.
Discussion
Since the 1990s minimally invasive cardiac surgery has generated great interest. In recent years the improvement in anesthesia, surgical techniques, myocardial protection, and postoperative practice allowed to treat aged patients with an increased surgical risk [5]. The main direction in cardiac surgery is to reduce the invasiveness of treatments, so many centers adopted MIAVR (minimally invasive aortic valve repair) as an alternative to full sternotomy [5].
The main advantage of minimally invasive surgery is shorter time of mechanical ventilation, ICU stay, and hospitalization. Also, there is less blood and plasma transfusion, bleeding, wound infection, pneumonia, and sternal instability. In our study we also observed shorter times of CPB and cross-clamp in the group where sutureless valve was implanted. The median time of hospitalization was equal for both groups, and it was 8 days. We did not observe any wound infection and sternal instability.
Surgery procedures
Filip et al. in the meta-analysis reported longer time of the procedure, ACC and CPB. The authors implanted standard biological prostheses. In our study, times of cross-clamp and CPB was significantly shorter in sutureless valve implantation versus mechanical prosthesis implanted from ministernotomy. This difference may arise because of implantation of rapid deployment sutureless valves (Perceval™ and Edwards Intuity™) [6–9]. Median cross-clamp time was 49 minutes (27; 84) and CPB 70 (40; 188) for sutureless valve, versus 69 (50; 103) cross-clamp time, and 95 (170; 69) for CPB time. In our opinion, use of sutureless valves is recommend to ministernotomy approach because of easy implantation especially in difficult surgical and anatomical conditions. The use of minimally invasive approach may be important in a group of patients with previous cardiac operations, especially patients with the CABG history. Tabata et al. reported that in a group of 146 patients who underwent reoperation with minimal access to aortic valve surgery, 93 had a left internal thoracic artery graft which remained undissected [4].
Post-operative course
30-day mortality was 2.5% in group I vs. 2.7% in group II. In addition, one patient from group I died after 45 days of hospitalization. According to Szecel’s study, 30-day mortality rate after Perceval valve implantation was 3.2% with mean EuroSCORE II of 5.1% [10]. This difference in early mortality could be a result of the type of surgery. In this study, more than half of cases was combined surgery. According to Kaplan-Meier probability of survival in our groups, annual, 2-year and 5-year probabilities of survival were 89% vs. 97%: 85% vs. 97% and 75% vs. 94%, in group I and II, respectively (p = 0.042) (Figure 1).
Our results are comparable with other authors. Shrestha et al. in a retrospective study reported 7% mortality in a group of 731 patients in 5 years’ follow-up duration [11]. Also, in Meuris’ study there was 71.3% overall 5-year survival [12]. In both studies the mean age, EuroSCORE and other risk factors of patients in whom Perceval valves were implanted, were significantly higher than in a group of patients with mechanical prosthesis implantation.
Patients who qualified to Perceval valve implantation, are a specific group of high-risk elderly patients, with a high rate of EuroSCORE. Moreover, patients which were qualified to sutureless valve implantation, were boundary patients between standard AVR or TAVI. There are studies where the mortality rate, incidence of PVL, stroke and myocardial infarction were all higher in the TAVI group [13–15].
According to Di Bacco, patients undergoing AVR with upper ministernotomy had shorter assisted ventilation time, faster recovery, and hospital discharge [8]. Also, a meta-analysis by Brown et al. showed that ventilation time was shorter in less-invasive procedures (differences of 2.1 hours) [2]. In our study 39 (51%) patients from group I had intubation time shorter than 24 hours. Only 1 (1%) patient needed prolonged intubation due to a severe general condition. In the group with conventional valve implantation, the prolonged intubation concerned 2 patients: one with bleeding and one with cardiogenic shock. The same authors showed that ICU and hospitalization time was shorter in the ministernotomy group [2]. In our study, median hospitalization time was 8 days for both groups.
Atrial fibrillation is the most common post-operative complication and most common arrhythmia after cardiac surgery [14]. There are conflicting reports of post-operative atrial fibrillation in patients after miniAVR. According to Gilmanov et al., after ministernotomy AVR in 28.5% of cases, new onset of AF was reported [15]. On the other hand, Shehada et al. showed in the meta-analysis that AF was present in 11.7% of patients after miniAVR [16]. According to their reports, a possible explanation is less manipulation of the heart in cases of minimal invasive approach [16]. In our study, atrial fibrillation occurred in 31 (41%) patients, of which 12 cases (15%) had AF before surgery.
The incidence of postoperative renal complications from minimally access AVR and conventional AVR is still controversial [15]. Shehada et al. reports in the meta-analysis a nonsignificant lower rate of postoperative renal injury in the MAAVR [16]. In our study, acute renal injury was present in 12 (15%) cases, 9 (22.5%) cases in group I and 3 (8.3%) in group II (p = 0.11). Four patients needed CVVH. One patient needed to CVVH course prior to surgery due to a history of kidney transplantation. In both groups, the risk of AKI was present/occurred in cases with a high level of creatinine before surgery. Patients in group I were also patients with a higher risk of surgery with median Euro-SCORE (1.48 vs. 0.57), lower GFR rate (74 ml/min/1.73 m2 vs. 105 ml/min/1.73 m2) and significantly higher median age (72 vs. 51). In our opinion, that could explain a higher rate of AKI in group I.
In Fattouch et al. study, they reported that blood transfusion was needed in 40.6% of patients operated by ministernotomy approach [17]. In our study, the median rate of red cell concentrate was 2 units. Also, frozen plasma was transfused in 52 (68%) cases with median rate of 1 unit. In our opinion, blood and plasma transfusion is lower in patients with minimally invasive approach, and it follows from less sternum trauma, and also use of sutureless and rapid deployment valves reduces extracorporeal circulation and aortic cross-clamp time and leads to less blood transfusion.
Deep wound (mediastinitis) infection occurs in 1–4% with average mortality of 10–25% [18]. The main risk factors are chronic lung disease, insulin-dependent diabetes mellitus, obesity, sternal osteoporosis [18]. In this study, we had 19 (19%) patients with diabetes. In our group of patients there was no case of wound infection or sternum instability because of wound infection. In our opinion, a small operative field and small incision reduces the risk of wound infection, also small sternum incision reduces the risk of instability and would result in less postoperative pain.
Permanent pacemaker implantation is required in 3–8% of all patients undergoing AVR [19]. Our study included 8 patients requiring PPM implantation in the group after sutureless valve implantation, compared with 3 patients in group II (p = 0.19). According to Shehada et al. meta-analysis, in 3.3% of miniAVR cases, PPM implantation was needed [18], also Gilmanov reports that 2.7% of patients were requiring PPM implantation. Vogt et al. study showed that patients who underwent sutureless AVR have a higher risk of AV block [20]. In our opinion, use of rapid deployment prosthesis especially after ballooning could predispose to AV block. Also according to Szecel et al., oversizing of the sutureless valve could be a reason of AV block because of excessive force on the left ventricular outflow tract, and the nearby conduction system [21]. We did not observe any relationship between minimally invasive approach and AV block.
Chronic lung disease is one of the main factors of sternum dehiscence [22], and prolonged mechanical ventilation after open heart surgery [23]. According to Bignami et al., full sternotomy and sternosynthesis are burdened with complications like presence of postoperative pain, which can affect breathing by decreasing the protective coughing reflex [23]. The same authors reported that incidental injury of phrenic nerve with a rate from 1% to 60% may affect diaphragm dysfunction and can increase pulmonary postoperative complications [23]. It can be the major risk factor for prolonged mechanical ventilation, hospitalization, and risk of infection in patients with chronic lung disease. In both our groups there were 8 (10%) patients with chronic lung disease. In our study, there was no case with sternal dehiscence, and no patients with chronic lung disease who needed prolonged ventilation.
Postoperative delirium is a common complication of cardiac surgery associated with increased mortality, morbidity, and long-term cognitive dysfunction [24]. The pathophysiology of delirium is not fully elucidated, but a common pathway lies in neuroinflammation [24]. Kotfis et al. reports that in a group of patients above 65 years of age, after valve repair there were 41 cases of postoperative psychosis [24]. In our study, in 7 males and 1 female (all from group I) there was incidence of postoperative delirium. According to Kotfis et al. study, there is a relation between time of CPB and cross-clamp and the frequency of delirium in the postoperative course, also age > 65 years old affects the frequency of psychosis. In our group, there was no relation between length of CPB and cross-clamp. Moreover, in group II where cross-clamp and CPB times were longer, we did not observe any episode of postoperative psychosis. The median age in cases with delirium was 71, there was 1 patient under 65 years old. In our opinion, not only the patient age, time of cross-clamp, and CPB affect the psychosis episode after cardiac surgery, but also factors like general condition or comorbidities.
Limitations: We are aware that the current study has several limitations. First, the retrospective non-randomized design and analysis of a limited number of patients from a single center reduces the statistical power of the study. The mortality and morbidity rate are very low as well as general favorable outcomes. Therefore, they may not reflect the results of other centers. Moreover, we must stress that this report was focused predominantly on mortality and morbidity and quality of life of survivors was not estimated. The latter aspect would increase markedly the significance of this study, therefore we plan to conduct such study in the very close future.
Conclusions
In our opinion, the ministernotomy approach for AVR gives a major benefit for high-risk patients especially with obesity or chronic lung disease. Moreover, the ministernotomy approach with sutureless valve implantation could shorten the cross-clamp and CPB time, which is an important factor for high-risk patients.