Introduction
Surgical aortic valve replacement (SAVR) and transcatheter aortic valve implantation (TAVI) are the treatments of choice for patients with symptomatic severe aortic stenosis. Patients with this kind of valve disorder are often at an advanced age and mostly have significant comorbidities, especially in patients with a previous history of myocardial infarction receiving coronary artery bypass grafting [1]. Thus, SAVR is often technically challenging in such high-risk patients even though SAVR has achieved excellent results [1]. The first-time TAVI in a human was performed in April 2002 by Cribier et al. [2] via an antegrade transseptal approach. The feasibility and results of both short- and intermediate terms of the TAVI by using Sapien Edwards and Medtronic Core Valve prostheses have been illustrated [3]. Observations indicated that TAVI had fewer vascular complications and lethal hemorrhagic events than SAVR [4]. Apart from the superior short- and intermediate-term outcomes of TAVI, clinical studies also showed that TAVI was at least as good as SAVR in terms of all-cause mortality or major stroke [5, 6]. Nevertheless, little was known about the comparative results of postoperative cerebral events and mortality between TAVI and SAVR for elderly patients with surgical/interventional indications.
The purpose of the present study was to make a comparison of the neurological events and mortality between patients receiving TAVI and SAVR.
Methods
Literature was retrieved from the databases, such as PubMed, Google Scholar and “Baidu” Scholar for publications of 2000–2022. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement guidelines were followed in this review. The used search terms and keywords included “surgical aortic valve replacement (SAVR)”, “transcatheter aortic valve implantation (TAVI)”, “symptomatic severe aortic stenosis”, “complications”, “cerebral events”, “stroke”, “all-cause mortality”, and “cardiovascular mortality”. The inclusion criterion was randomized controlled trials. The primary exclusion criteria were publications reporting TAVI: lack of patient information (n = 4), with no information of patient outcome (n = 1), with an observational time for outcomes of over 1 year (n = 2), and a case report other than prospective research (n = 1); focusing health status benefits (n = 4), cost-effectiveness (n = 3), major vascular and bleeding complications (n = 3), prosthesis-patient mismatch (n = 3), postoperative atrial fibrillation (n = 2), heart function (n = 2), impact of preoperative moderate/severe mitral regurgitation (n = 1), length of stay (n = 1), effect of baseline aortic regurgitation on mortality (n = 1), impact of transfusion (n = 1), post-TAVI paravalvular leak (n = 1), procedure safety evaluations (n = 1), and sex-related outcomes (n = 1), instead of focusing on postprocedural neurological outcomes and (or) mortalities. Consequently, 10 articles were included in this study and 32 were excluded. The inclusion and exclusion of literature retrieval are shown in Figure 1.
Statistical analysis
IBM SPSS Statistics version 26 software was used to perform statistical analysis. The measurement data were expressed as mean ± standard deviation and were compared by the independent t test, and the categorical data were expressed as numbers and percentages and compared by the χ2 test. Review Manager 5.4.1 was used to draw forest plots. P < 0.05 was considered statistically significant.
Results
In total 10 prospective research publications [1, 7–15] were included in this study, with data on 5,969 patients. There were 3,073 (51.5%) and 2,896 (48.5%) patients in the TAVI and SAVR groups, respectively. Patients were identified as high-risk by the heart team if their estimated 30-day mortality risk was ≥ 15% in the absence of extreme risk [1, 13]. Risk was assessed using the Society of Thoracic Surgeons Predicted Risk of Mortality score and other characteristics associated with increased surgical risk [1], whereas they were regarded as non-high-risk when the estimated 30-day mortality risk was < 15%. Of the 5,969 patients, 1,154 (19.3%) were high-risk patients, with 595 (51.6%) and 559 (48.4%) high-risk patients in TAVI and SAVR groups, respectively. Gender was known for 5,328 patients with 3,242 (60.8%) male and 2,086 (39.2%) female patients. The male-to-female ratio was 1.6 : 1. There were 1,673 (61.0%) and 1,569 (60.7%) male patients, respectively, in the two groups (χ2 = 0.1, p = 0.80). Patients were at an average age of 79.6 ±2.8 (range: 73.7–82.9; median: 80.5) years. No difference was found in patient age between the two groups (79.3 ±2.8 years vs. 79.9 ±2.9 years, t = 0.460, p = 0.85), or in body surface area (1.9 ±0.4 kg/m2 vs. 1.9 ±0.5 kg/m2, t = 0.283, p = 0.78). The frequencies of preoperative comorbidities did not show intergroup differences (Table I).
Table I
Intergroup comparison of preoperative comorbidities and postoperative events
| Variable | Transcatheter aortic valve implantation (n = 3,073) | Surgical aortic valve replacement (n = 2,896) | χ2 | P-value |
|---|---|---|---|---|
| Preoperative comorbidity: | ||||
| Diabetes mellitus | 932/2,466 (37.8) | 907/2,303 (39.4) | 1.3 | 0.27 |
| Renal disease, creatinine > 2 mg/dl | 109/1,601 (6.8) | 115/1,480 (7.8) | 1.1 | 0.33 |
| Cardiovascular disease | 550/2,745 (20.0) | 516/2,596 (19.9) | 0.0 | 0.86 |
| Peripheral vascular disease | 719/2,615 (27.5) | 659/2,441 (27.0) | 0.2 | 0.71 |
| Stroke | 154/1,443 (10.7) | 153/1,344 (11.4) | 0.4 | 0.59 |
| Transient ischemic attack | 95/1,162 (8.2) | 82/1,077 (7.6) | 0.2 | 0.64 |
| Atrial fibrillation | 1,247/2,500 (49.9) | 1,191/2,330 (51.1) | 0.7 | 0.40 |
| Myocardial infarction | 235/1,487 (15.8) | 221/1,396 (15.8) | 0.6 | 0.44 |
| Chronic obstructive pulmonary disease | 799/2,432 (32.9) | 744/2,271 (32.8) | 0.0 | 0.95 |
| Coronary artery bypass grafting | 584/2,470 (23.6) | 559/2,306 (24.2) | 0.2 | 0.64 |
| Percutaneous transluminal coronary angioplasty | 381/1,615 (23.6) | 336/1,508 (22.3) | 0.8 | 0.40 |
| Valve surgery | 3/115 (2.6) | 1/111 (0.9) | 0.9 | 0.62 |
| Balloon valvuloplasty | 54/595 (0.2) | 44/559 (7.9) | 0.5 | 0.53 |
| Postoperative events (overall): | ||||
| 1-month major stroke | 84/2,615 (3.2) | 91/2,441 (3.7) | 1.0 | 0.32 |
| 1-month all-cause mortality | 51/2,470 (2.1) | 63/2,311 (2.7) | 2.2 | 0.16 |
| 1-month cardiovascular mortality | 27/876 (3.1) | 29/831 (3.5) | 0.2 | 0.68 |
| 1-year all-cause mortality | 191/2,209 (8.6) | 248/2,100 (11.8) | 11.8 | 0.001 |
| 1-year cardiovascular mortality | 79/1,334 (5.9) | 91/1,286 (7.1) | 1.4 | 0.24 |
| Postoperative events (non-high-risk): | ||||
| 1-month major stroke | 53/2,020 (2.6) | 60/1,882 (3.2) | 1.1 | 0.30 |
| 1-month all-cause mortality | 19/1,875 (1.0) | 25/1,752 (1.4) | 1.3 | 0.29 |
| 1-month cardiovascular mortality | 3/281 (1.1) | 6/272 (2.2) | 1.1 | 0.33 |
| 1-year all-cause mortality | 83/1,614 (5.1) | 130/1,541 (8.3) | 13.6 | < 0.001 |
| 1-year cardiovascular mortality | 25/739 (3.4) | 31/727 (4.3) | 0.8 | 0.42 |
| Postoperative events (high-risk): | ||||
| 1-month major stroke | 31/595 (5.2) | 31/559 (5.5) | 0.1 | 0.90 |
| 1-month all-cause mortality | 32/595 (5.4) | 38/559 (6.8) | 1.0 | 0.33 |
| 1-month cardiovascular mortality | 24/595 (4.0) | 23/559 (4.1) | 0.0 | 1.00 |
| 1-year all-cause mortality | 108/595 (18.2)* | 118/559 (21.1)** | 1.6 | 0.21 |
| 1-year cardiovascular mortality | 54/595 (9.1) | 60/559 (10.7) | 0.9 | 0.38 |
All patients were diagnosed with symptomatic severe aortic stenosis and referred for TAVI or SAVR. The TAVI prosthetic valve type was reported for 2,495 patients: a self-expanding prosthesis was used for 2,037 (81.6%) patients and any valve with a CE mark was used in 458 (18.4%) patients. The types of valves in the SAVR group were only reported in one of the included reports, which were stented xenograft (n = 411), stentless xenograft (n = 3) and mechanical valve prostheses (n = 2) [15]. The sizes of valves in SAVR were reported for 658 patients in 3 publications [13–15]. In total, valve sizes were known for 658 patients receiving SAVR: #19 (n = 72, 10.9%), #21 (n = 165, 25.1%), #23 (n = 255, 38.8%), #25 (n = 135, 20.5%), #27 (n = 25, 3.8%), #29 (n = 5, 0.8%), and #31 (n = 1, 0.2%). The percutaneous routes for TAVI were reported for 889 (34%, 889/2,615) patients of this patient cohort, with transfemoral access in 797 (89.7%), transapical access in 70 (7.9%), transsubclavian access in 15 (1.7%) patients, and a direct aortic route in 7 (0.8%) patients.
The duration of the procedure (86.2 ±5.9 min vs. 179.6 ±3.4, p = 0.003), and the hospital stay (5.3 ±1.9 days vs. 9.4 ±2.0 days, p = 0.010) were much shorter, and the number of transfused blood units was much lower (2.8 ±3.1 units vs. 0.4 ±1.1 units, p < 0.001) in the TAVI than in the SAVR group. The ICU stay (60.4 ±17.6 h vs. 81.0 ±39.7 h, p = 0.46) was shorter in the TAVI group but without statistical significance.
The postoperative stroke rate did not show an intergroup difference (Table I). No intergroup differences were noted in 1-month all-cause mortality (p = 0.16), 1-month cardiovascular mortality (p = 0.684) or 1-year cardiovascular mortality (p = 0.24), without much intergroup differences, whereas 1-year all-cause mortality (p < 0.001) was much lower in the TAVI than the SAVR group with a very significant difference (Table I). In the forest plots, there was no heterogeneity between studies for 1-month all-cause mortality, 1-month and 1-year cardiovascular mortality. However, there was moderate heterogeneity for 1-year all-cause mortality with an I2 = 52%. The test for the overall effect for 1-year all-cause mortality showed z = 3.64, p = 0.0003, which was < 0.05, indicating statistical significance for the overall effect (Figures 2–5).
Figure 2
Forest plots for 1-month all-cause mortality
SAVR – surgical aortic valve replacement, TAVI – transcatheter aortic valve implantation.
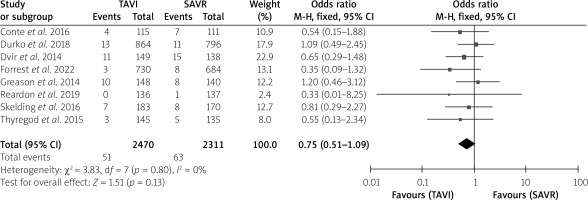
Figure 3
Forest plots for 1-month cardiovascular mortality
SAVR – surgical aortic valve replacement, TAVI – transcatheter aortic valve implantation.
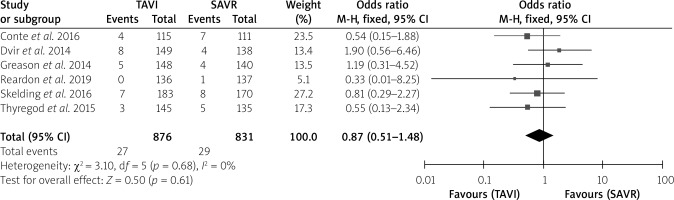
Figure 4
Forest plots for 1-year all-cause mortality
SAVR – surgical aortic valve replacement, TAVI – transcatheter aortic valve implantation.
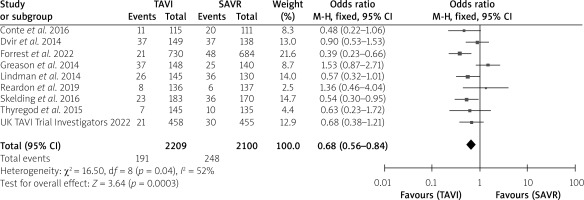
Figure 5
Forest plots for 1-year cardiovascular mortality
SAVR – surgical aortic valve replacement, TAVI – transcatheter aortic valve implantation.
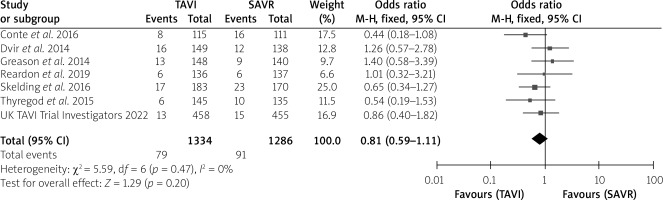
Data of postoperative neurological events and mortality are divided into “non-high-risk” and “high-risk” subgroups. Statistical analyses revealed that, in the non-high-risk subgroup, the 1-year all-cause mortality was much lower in TAVI than in SAVR patients (5.1% vs. 8.3%, p < 0.001); and comparisons between non-high-risk and high-risk subgroups revealed that the 1-year all-cause mortality of TAVI was much lower in the non-high-risk than the high-risk subgroup (5.1% vs. 18.2%, p < 0.001), and the 1-year all-cause mortality of SAVR was also much lower in the non-high-risk than the high-risk subgroup (8.3% vs. 21.1%, p < 0.001) (Table I). Therefore, subgroups of risk stratification showed better outcomes for non-high-risk patients compared with high-risk patients, and also better outcomes for TAVI patients than SAVR patients.
Discussion
The ACC/AHA strongly recommends SAVR for patients younger than 65 years or life expectancy expected to exceed 20 years, and TAVI for patients older than 80 years or life expectancy less than 10 years, whereas ESC/EACTS Guidelines recommend that SAVR be performed in younger patients < 75 years at low risk for a surgical operation or in patients for whom a surgical operation other than TAVI is indicated, and TAVI is recommended in older patients ≥ 75 years, or in patients at high risk or unsuitable for a surgical operation [16]. In accordance with the current ESC and EACTS guidelines, TAVI is preferred for patients aged ≥ 75 years, or at a high risk (STS-PROM/EuroSCORE II > 8%), or not suitable for a surgical operation.
TAVI patients require fewer blood transfusions, suggesting a decreased incidence of perioperative bleeding complications. As reported by Conte et al. [1], the lengths of both hospitalization and ICU stay were significantly reduced, and procedural duration was much shorter in the TAVI group, indicating the important role of TAVI in reducing the surgical trauma and the cost of the procedure [1].
Biologic heart valves carry the risk of deterioration, whereas mechanical heart valves, compared with bioprostheses, are less prone to structural deterioration and need lifelong anticoagulants to prevent thromboembolic events [17, 18]. Comparisons between mechanical and biological prostheses produced heterogeneous results. Bruscky et al. [19] reported that the overall mortality-free and reoperation-free survival rates were much higher in patients with a mechanical prosthesis than in those with a biological one. Meanwhile, patients of both groups showed similar composite adverse event risks. The hemorrhagic risk was much higher in patients with a mechanical prosthesis. Yu et al. [20] reported that patients > 70 years undergoing mechanical mitral valve replacement had lower long-term mortality and a 20% greater risk of stroke or systemic embolism compared with patients undergoing biological mitral valve replacement. The types and sizes of aortic valve prostheses were determining factors influencing left ventricular mass following aortic valve replacement. The mean transvalvular gradients decreased and the effective orifice area as well as the index of it increased with increasing valve size for both mechanical and biologic prostheses [21]. A small aortic prosthesis for aortic stenosis may cause slow reversal of left ventricular mass, and increase long-term mortality and valve-related complications [22]. The smaller sized valves of #19–23 may lead to an increased risk of death [23]. Patients with prosthesis-patient mismatch were associated with lower stroke-free survival compared with prosthesis-patient mismatch-free patients during a mean follow-up of 34 months after TAVI (81% vs. 94%, p = 0.05) [24].
Studies have demonstrated that TAVI had better neurological outcomes and survival compared with SAVR. Conte et al. [1] found similar all-cause or cardiovascular mortality at 30 days (TAVI 3.5% vs. SAVR 6.3%; p = 0.33). At 1 year, the TAVI patients had improved survival with significant reduced all-cause and cardiovascular mortality rates than the SAVR patients. The observed-to-expected mortality ratio predicted risk of death was < 1 in both TAVI and SAVR groups (0.48 vs. 0.79), indicating better than expected outcomes [1]. Univariant analysis revealed that predictive risk factors of short- and long-term mortalities were European System for Cardiac Operative Risk Evaluation score > 20% (p = 0.04), peripheral vascular disease (p = 0.03), and left ventricular diastolic dysfunction (p < 0.01). Patients at the age of or younger than 80 years and Society of Thoracic Surgeons risk score were not significant predictors [1].
In diabetic patients, stroke rates at 30 days and 1 year were similar between TAVI and SAVR [11]. Eggebrecht et al. [25] reported that the stroke/transient ischemic attack rate of patients receiving TAVI at 30 days was 3.3%. The strokes were mostly major strokes and the mortality was high. In the present study, the 1-month major stroke rate was 3.2%, which was very close to the result reported above. Zahn et al. [3] evaluated cerebral events and the outcomes of TAVI patients between those with and those without a porcelain aorta, and found no intergroup differences concerning the in-hospital mortality or stroke.
In diabetic patients, both 6-month and 1-year all-cause mortality rates were significantly lower in the TAVI than the SAVR group, whereas at 2 years, the survival rates did not differ between TAVI and SAVR. In comparison, in nondiabetic patients, the 1-year all-cause mortality of TAVI was similar to that of SAVR. Nevertheless, transapical TAVI seemed to have increased mortality compared with SAVR. Moreover, nondiabetic patients with TAVI carried a higher stroke rate than SAVR at 1 year (7.6% vs. 2.8%, p = 0.06) [11].
The postoperative outcome observation revealed significantly reduced incidences of 1-year major adverse cardiovascular and cerebrovascular events (17.5% vs. 28.1%, p = 0.05), 1-year neurologic events (20.2% vs. 32.4%, p = 0.06), major stroke (6.6% vs. 8.8%, p = 0.54), and obvious hemorrhage (33% vs. 48%, p = 0.017) in the TAVI than in the SAVR group [1]. The CoreValve High Risk study confirmed that TAVI patients with the use of the self-expanding CoreValve bioprosthesis had improved 1-year survival compared with SAVR [26]. This result was supported by the present study.
However, some retrospective studies have found no survival benefits with TAVI or SAVR in patients with a previous history of coronary artery bypass grafting [27]. Nguyen et al. [28] reviewed information of 107 TAVI and 148 SAVR patients, and found that TAVI patients showed better early survival and similar mid-term survival.
Long-term observations illustrated that all-cause mortality rates at 5- [29–31] and 8-year follow-ups [5], and the cardiovascular mortality at 8-year follow-up [5] were similar in TAVI and SAVR groups.
The study showed better results in the subgroup of non-high-risk patients after TAVI compared to non-high-risk patients after SAVR. However, no significant differences were found in high-risk patients in terms of postoperative neurological events and mortalities. As Ray [32] indicated, TAVI was associated with better left ventricular ejection fraction recovery than SAVR when discharged and 1 year after the procedure for nearly three times more patients receiving TAVI had a normal left ventricular ejection fraction (> 50%). This might be explained by the better valve hemodynamics, lower risk of prosthesis–patient mismatch, and avoidance of cardiopulmonary bypass for TAVI patients. We can see in Table I the outcomes of high-risk patients, which showed that the TAVI group had a slightly better result than the SAVR group, but without a significant difference. It might be explained by the incomplete information of the study materials. Thus, further randomized controlled trials are needed to confirm the superiority of TAVI over SAVR in high-risk patients.
There were limitations in the present study. Although all the study materials were obtained from prospective randomized trials, biases still exist in the comparative results of TAVI versus SAVR in patients with severe aortic stenosis. The majority of TAVI procedures included in this study were lacking detailed information of types and sizes of implanted aortic valve prostheses. Patients undergoing TAVI receiving different prostheses may experience various clinical results, but evaluations on these aspects were not practical. Finally, patient data of the percutaneous route of TAVI were not available in detail.
In conclusion, TAVI might be associated with reduced 1-year all-cause mortality. The subgroups of risk stratification revealed better outcomes for non-high-risk patients than for high-risk patients, and also better for TAVI patients than for SAVR patients. Thus it is recommended that elderly patients with symptomatic severe aortic stenosis choose TAVI for treatment.









