Introduction
Gastric cancer (GC) is a prevalent malignancy affecting the digestive tract, and individuals diagnosed with this condition commonly experience nutritional deficiency, which can be further exacerbated by surgical treatment [1]. Malnutrition is a contributing factor that has been associated with the suppression of immunological function, alteration in inflammatory response, and amplification of stress response [2]. Consequently, these patients frequently experience poor surgical outcomes in several domains, including post-operative infections and complications, systemic inflammation, wound healing failure or delay, and prolonged hospital stay [3, 4]. From a nutritional perspective, parenteral or enteral feeding supplements have been suggested as a crucial adjuvant treatment for postoperative patients and are selected as per the individual patient’s gastrointestinal function and ability to tolerate certain nutrient delivery methods [5].
Enteral nutrition (EN) is favoured due to its alignment with physiological characteristics and its association with reduced post-operative complications and costs. However, despite the provision of essential nutrients such as energy, protein, fat, carbohydrates, minerals, vitamins, etc., the effects of EN have been found to be less significant than originally expected [6]. Consequently, there has been increasing scientific concern with the utilization of enteral immunonutrition (EIN), which involves the incorporation of ω-3 fatty acids, glutamine (Gln), arginine (Arg), and nucleotide. The utilization of EIN has been recognized as a noteworthy therapeutic strategy in mitigating surgical infection and non-infectious complications, augmenting host immunity, and ameliorating patient prognosis in instances of gastrointestinal malignancy [7].
The primary constituents of EIN consist of arginine, a semi-essential amino acid that plays a crucial role in various cellular metabolic processes, glutamine, an essential nutrient necessary for the metabolism of intestinal mucosal cells, nucleotides such as RNA, and omega-3 fatty acids (ω-3-FAs), which exhibit immunomodulatory and anti-inflammatory properties [8, 9]. The supplementation of these components is crucial due to the rapid depletion of these components in the intestinal mucosal epithelial cells during periods of heightened stress, such as surgical operations or infections. This depletion ultimately results in a weakened immune response inside the intestines [10].
Recent studies have demonstrated that EIN is more effective in enhancing the immune response of GC patients who have had gastrectomy, when compared to EN. The enhancement of immune activity eventually results in improved patient outcomes [11, 12]. Nevertheless, some studies have failed to reliably establish its therapeutic advantages [13, 14]. However, there is inconsistency in the results that have been reported because of the heterogeneity seen between studies due to differences in demographics, nutritional condition, and research duration.
Hence, the main objective of this meta-analysis was to assess and evaluate the impact of supplementing conventional EN with EIN on various biochemical, immunological markers, and clinical outcomes in individuals who have undergone surgical intervention for GC.
Aim
This study aims to compare the effects of incorporating EIN into standard EN on a range of biochemical, immunological markers, and clinical outcomes in patients who have undergone surgery for GC, as well as evaluation of evidence networks pertaining to EIN formulae.
Material and methods
Search strategy
The present meta-analysis, with registration number WU#/IRB/2023/5040, was conducted following a comprehensive search across various databases, including Medline (via PubMed), Embase, Scopus, Cochrane Library, and Web of Sciences. The search covered the period from the year 2000 to 2023 and utilized specific keywords such as “enteral immunonutrition”, “enteral nutrition”, “gastric cancer”, “total gastrectomy”, “post-operative infections”, “post-operative complications”, “post-operative systemic inflammation rate”, “immune and inflammatory factors”, “cellular immunity”, and “serum proteins”. Based on the PICOs framework, the keywords were identified and found to be consistent in both the Medline and EMBASE databases, as indicated in Table I. In the context of searching Scopus, the Title (ti)-Abstract (abs)-keyword (key) field was utilized with the aforementioned keywords. The key phrase “enteral immunonutrition” was utilized in the Cochrane database.
Table I
Database search strategy
| Database | Search strategy |
|---|---|
| Scopus | #1 “enteral immunonutrition” OR “enteral nutrition” OR “gastric cancer” OR “total gastrectomy” #2 “post-operative infections” OR “post-operative complication” OR “post-operative mortality rate” OR “immune and inflammatory factors” OR “Cellular immunity” OR “Serum proteins” #3 #1 AND #2 |
| PubMed | #1 “enteral immunonutrition” OR “enteral nutrition” [MeSH Terms] # OR “gastric cancer” [All Fields] OR “[All Fields]” OR “total gastrectomy” [All Fields] #2 “post-operative infections” [MeSH Terms] OR “post-operative complication” [All Fields] OR “post-operative mortality rate” [All Fields] OR “immune and inflammatory factors” [All Fields] OR “Cellular immunity” OR “Serum proteins” [All Fields] #3 #1 AND #2 |
| Embase | “Enteral immunonutrition”/exp$ OR “enteral nutrition”/ exp OR “gastric cancer”/exp OR “Total gastrectomy”/exp #2 “post-operative infections”/exp OR “post-operative infectious complications”/exp OR “post-operative mortality”/exp OR “cellular immunity”/exp OR “immune and inflammatory factors”/exp OR “Serum proteins”/exp #3 #1 AND #2 |
| Cochrane library | #1 (enteral immunonutrition): ti, ab, kw@ OR (gastric cancer): ti, ab, kw OR (gastric surgery): ti, ab, kw OR (total gastrectomy): ti, ab, kw OR (enteral nutrition): ti, ab, kw (Word variations have been searched) #2 (post-operative infection): ti, ab, kw OR (post-operative infectious complication): ti, ab, kw OR (post-operative mortality): ti, ab, kw or (cellular immunity): ti, ab, kw or (immune and inflammatory factors): ti, ab, kw or (Serum proteins): ti, ab, kw (Word variations have been searched) #3 #1 AND #2 |
The PICO format was utilized to construct precise selection criteria. In this context, “P” denoted gastric cancer patients having gastrectomy, “I” referred to enteral immune nutrition, “C” denoted standard enteral nutrition, and “O” encompassed clinical outcomes, immunological factors, and nutritional status indices. The design methodology employed in this study was confined to the utilization of randomized controlled trials (RCTs). The inclusion criteria specified that only papers published in English language were considered. The inclusion of articles was conducted in accordance with the principles outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15]. Two researchers, identified as LH and QZ, independently conducted a comprehensive review of the pertinent literature to identify relevant studies.
Inclusion and exclusion criteria
The present analysis encompassed studies that provided information on the comparative utilization of EIN and EN in the context of surgical interventions for patients with GC. The selection of studies encompassed the period from 2000 to 2023. We selected studies that had full text and provided adequate data for a 2 × 2 table. This meta-analysis incorporated various clinical outcomes as primary measures, including the occurrence of post-operative infections, post-operative complications, systemic inflammation, postoperative infectious complications, and immunological factors such as T-cell subsets (specifically the ratio of CD4+ and CD8+ cells) and lymphocyte count. Furthermore, serum protein components, namely proalbumin and transferrin, were also considered. References that were outdated, anecdotal, or based on expert opinions, as well as non-randomized controlled trials, experimental data from animal studies or trials, and studies whose primary data and important information from authors could not be obtained, were omitted. Additionally, studies that included GC patients together with those diagnosed with other types of malignancies, and papers published in languages other than English were also deleted. The researchers (LH and QZ) separately gathered demographic profiles of the patients and event data with significant factors from the included studies [16–25].
Quality assessment of included studies
A pre-established standardized questionnaire assessed possible bias in the papers analysed. The risk of bias was assessed using a Cochrane Collaboration tool [26] that was published in the Cochrane Handbook (version 5.3). The tool included 7 items: generating random sequences, concealing allocations, blinding personnel and participants, blinding outcome assessors, selective reporting, incomplete outcome data, and other biases. The assessment of potential bias was conducted separately by 2 reviewers, LH and QZ. A third reviewer, identified as WL, served as an arbitrator to resolve any remaining conflicts. Ultimately, the possible bias was assessed and categorized as either “high risk”, “low risk”, or “unclear risk”. The presence of publication bias was assessed using a funnel plot [27], and Begg’s test [28] was performed using the MedCalc software [29] to determine its statistical significance.
Statistical analysis
Review Manager (RevMan) 5.3 [30] software was utilized to assess and analyse the impact of several dichotomous and continuous outcomes. The utilization of reference management software facilitated the organization, extraction, and removal of duplicate references. Forest plots [31] were developed to assess the impact of outcome factors across all the investigations. The odds ratio (OR) was computed using the DerSimonian Lair method, utilizing a 2 × 2 table [32] constructed with event data. The evaluation of dichotomous outcomes involved the use of OR along with a 95% confidence interval (CI). The standard mean difference (SMD) with a 95% CI was utilized to represent the outcome data. Heterogeneity was assessed using statistical methods, including the χ2 test with a matching p-value and the I2 test [33]. If there was heterogeneity between studies, as indicated by an I2 value greater than 50% or a p-value less than 0.05, a random-effects model was employed. Otherwise, a fixed-effect model was used for the pooled analysis [34]. A p-value below 0.05 was deemed to be statistically significant [35].
Results
Literature search results
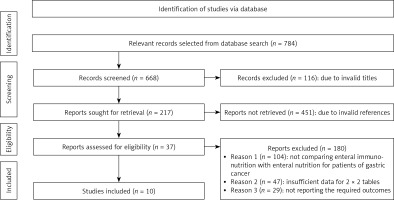
A comprehensive search of several databases was conducted using electronic scanning techniques, resulting in the finding of 784 studies that met the inclusion criteria outlined by the PICOS framework. A total of 116 papers were removed based on a thorough examination of their titles and abstracts, leaving us with 668 records that underwent further screening. Moreover, because of inadequate references and duplications, 451 studies were eliminated from consideration, leaving us with a final pool of 217 papers for further screening. A total of 217 studies were initially considered for inclusion in the analysis. However, after applying the inclusion-exclusion criteria, 180 studies were deemed ineligible and thus eliminated. The remaining 37 papers underwent further assessment to determine their eligibility for inclusion in the analysis. The primary factors contributing to the removal of studies were the absence of a comparison between EIN and EN for patients with GC, insufficient data to construct 2 × 2 tables, and the unavailability of necessary outcome measures. In this meta-analysis, a total of 10 papers meeting the specified inclusion criteria, which spanned the years from 2005 to 2022 were utilized, as seen in Figure 1. The studies included in this analysis encompass 1409 patients diagnosed with GC across various age cohorts. The selection of patients for this study was conducted using a random sampling method, and they were thereafter assigned to receive either an EIN or an EN intervention prior to undergoing gastrectomy. Table II displays the demographic characteristics of the studies used in this meta-analysis. The text provides a description of the publication’s journal, research type, country of study, patient diagnosis, age range of patients, sample size, elements of EIN, nature of EN, initiation time of EIN, duration of follow-up, manner of enteral feeding, and reported outcomes. Subsequently, the aforementioned event data were utilized to conduct the meta-analysis.
Table II
Brief summary of included studies
| Study ID | Publication year | Journal of publication | Type of study | Country | Diagnosis | Age of patients [years] | Sample size | Elements of EIN | Nature of EN | EIN initiation time | Follow up [days] | Mode of enteral feeding | Reported outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EN group | EIN group | EN group | EIN group | ||||||||||||
| Chen et al. [16] | 2005 | Asian Journal of Surgery | RCT | China | Gastric carcinoma | > 18 | > 18 | 20 | 20 | Lutamine, arginine, and omega-3 fatty acids | Standard EN | Post-operative | 9 | Nasoenteral | Plasma albumin, prealbumin and transferrin, immunoglobulin (Ig) A, IgG, IgM, CD4 and CD8 cell counts, the ratio of CD4/CD8, interleukin (IL)-2, IL-6 and tumour necrosis factor (TNF)-α |
| Farreras et al. [17] | 2005 | Clinical Nutrition | RCT | Spain | Gastric cancer | 69.2 ±13.8 | 66.7 ±8.3 | 30 | 30 | Arginine, omega-3 fatty acids and ribonucleic acid (RNA) | Standard EN | Pre-operation | 7 | Oral | Local hydroxyproline levels and episodes of surgical wound healing complications, rates of infectious complications, overall postoperative systemic inflammation |
| Fujitani et al. [18] | 2012 | The British Journal of Surgery | RCT | Japan | Gastric cancer | 65 ±14 | 64 ±12 | 111 | 120 | Arg and RNA | Regular diet | Pre-operation | 5 | Oral | Incidence of surgical-site infection (SSI), rates of infectious complications, overall postoperative systemic inflammation and C-reactive protein (CRP) levels on 3–4 days after surgery |
| Li et al. [19] | 2019 | Journal of Investigative Surgery | RCT | China | Gastric cancer | 63.1 ±11.6 | 62.9 ±10.4 | 62 | 62 | Arginine, glutamine, omega-3 fatty acids and nucleotide | Standard EN | Post-operative | 5 | Oral | CD4+ T-cells, CD3+ T-cells as well as counts of CD4+/CD8+, IgG, IgM, and IgA levels, white blood cell (WBC), C-reactive protein (CRP), procalcitonin (PCT), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) levels and nutritional index such as serum albumin, prealbumin, and transferrin concentration |
| Liu et al. [20] | 2012 | Journal of Digestive Diseases | RCT | China | Advanced Gastric cancer | 58.4 ±6.3 | 57.3 ±7.1 | 24 | 28 | Arginine and Glutamine (Gln) | Standard EN | Post-operative | 12 | Nasoenteral | Serum parameters including total protein, albumin, proalbumin and transferrin Levels of immunoglobulin M (IgM), immunoglobulin G (IgG), natural killer (NK) cells, CD4+ and CD8+ T cells |
| Marano et al. [21] | 2013 | Annals of Surgical Oncology | RCT | Italy | Gastric cancer | 65.1 ±10 | 66.6 ±11 | 55 | 54 | Arginine, omega-3 fatty acids and ribonucleic acid (RNA) | Standard EN | Post-operation | 20 | Oral | Postoperative infectious complications, anastomotic leak rate, and length of hospitalization, overall postoperative systemic inflammation, postoperative CD4+ T-cell counts |
| Mochiki et al. [22] | 2011 | World Journal of Surgery | RCT | Japan | Gastric surgery | 59 ±2.1 | 65 ±2.6 | 16 | 15 | Arginine and glutamine | Standard EN | Post-operation | 12 | Oral | Manometric recording 12 days after surgery, and plasma glutamine concentrations |
| Okamoto et al. [23] | 2009 | World Journal of Surgery | RCT | Japan | Gastric cancer | 70.9 ±13.2 | 66.9 ±11.5 | 30 | 30 | Arginine, glutamine and ω-3 fatty acids | Standard EN | Pre-operation | 7 | Oral | Incidence of postoperative infectious complications, duration of SIRS (systemic inflammatory response syndrome), postoperative lymphocyte and CD4+T-cell counts |
| Scislo et al. [24] | 2016 | Nutrition and Cancer | RCT | Poland | Gastric cancer | 60 ±3 | 64.5 ±2 | 44 | 54 | Glutamine, alanine, omega-3 fatty acids | Standard EN | Post-operation | 60 | Oral | Postoperative infectious complications, overall postoperative systemic inflammation complications and postoperative |
| Zhou et al. [25] | 2023 | British Medical Journal | RCT | China | Gastric cancer | > 18 | > 18 | 348 | 348 | Glutamine, alanine, omega-3 fatty acids | Standard EN | Post-operation | 90 | Oral | Long-term disease-free survival (DFS), postoperative CD4+T-cell counts postoperative complications and postoperative systemic inflammation |
Quality assessment
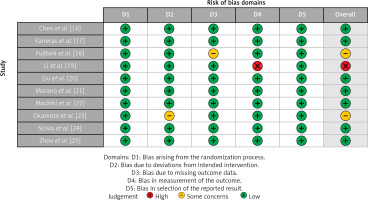
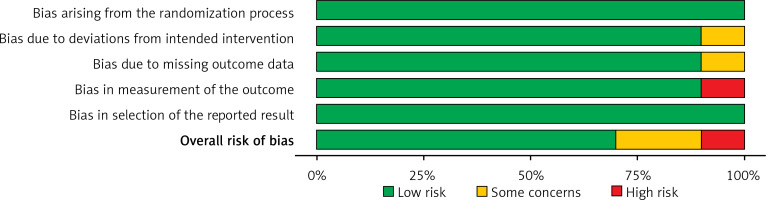
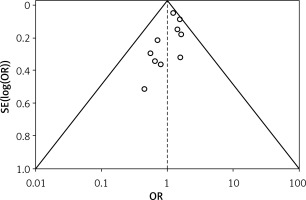
Table III displays the risk of bias assessment results for the included studies, based on the predetermined standardized criteria. The current meta-analysis has a minimal risk of bias, as shown by the Risk of Bias Summary (Figure 2) and the Risk of Bias Graph (Figure 3). Out of the 10 studies that were included in the analysis, 7 had a low risk of bias, while 2 were found to have a moderate risk of bias. The moderate risk was attributed to deviations from the intended intervention and missing outcome data. However, one study exhibited a significant risk of bias as a result of measuring issues pertaining to the outcome. Additionally, there was a minimal presence of publication bias, indicated from the symmetrical form of the funnel plot depicted in Figure 4 as well as the statistically insignificant p-value of Begg’s test (0.248, which is greater than the predetermined significance level of 0.05).
Table III
Risk assessment of included studies
| Variable | Chen et al. [16] | Farreras et al. [17] | Fujitani et al. [18] | Li et al. [19] | Liu et al. [20] | Marano et al. [21] | Mochiki et al. [22] | Okamoto et al. [23] | Scislo et al. [24] | Zhou et al. [25] |
|---|---|---|---|---|---|---|---|---|---|---|
| Did the study avoid inappropriate exclusions? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Did all patients receive the same reference standard? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were all patients included in the analysis? | N | N | N | N | N | N | N | N | N | N |
| Was the sample frame appropriate to address the target population? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were study participants sampled in an appropriate way? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were the study subjects and the setting described in detail? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were valid methods used for the identification of the condition? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Was the condition measured in a standard, reliable way for all participants? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
Findings from the statistical analysis
The present meta-analysis consisted of a sample of 10 randomized controlled trials, involving a total of 1409 GC patients. From the total population, 714 individuals were given EIN, whereas 695 persons were given standard EN. The statistical analysis was performed on the key outcomes of the study, yielding the subsequent findings.
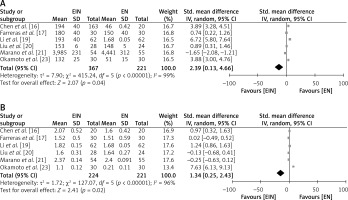
Post-operative levels of serum proteins of the EIN vs. EN group
To investigate the comparative impact of supplementing EIN with standard EN in GC patients, the postoperative levels of serum proteins proalbumin and transferrin were examined, as depicted in Figure 5. The EIN group had a high level of serum proalbumin with a SMD of 2.39 (95% CI: 0.13 to 4.66) with a τ2 value of 7.90, χ2 = 415.24, df = 5, Z = 2.07, I2 = 99%, and p = 0.04 (Figure 5 A). Similarly, the EIN group had a high level of serum transferrin with a SMD of 1.34 (95% CI: 0.25 to 2.43) with a τ2 value of 1.72, χ2 = 127.07, df = 5, Z = 2.41, I2 = 96%, and p = 0.02 (Figure 5 B).
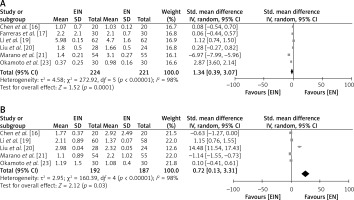
Post-operative immunological parameters of the EIN vs. EN group
To examine the comparative impact of supplementing EIN with standard EN in GC patients, the postoperative levels of the immunological parameters ratio of CD4+/CD8+ and lymphocyte counts were examined, as depicted in Figure 6. The EIN group had a high level of lymphocytes with a SMD of 1.34 (95% CI: 0.39 to 3.07) with a τ2 value of 4.58, χ2 = 272.92, df = 5, Z = 2.07, I2 = 98%, Z = 1.52, and p = 0.0001 (Figure 6 A). Similarly, the EIN group had a high ratio of CD4+/CD8+ with a SMD of 0.72 (95% CI: 0.13 to 3.31) with a τ2 value of 2.95, χ2 = 160.39, df = 4, Z = 2.12, I2 = 98%, and p = 0.03 (Figure 6 B).
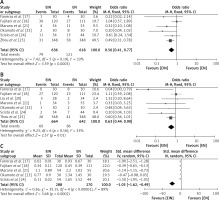
Post-operative clinical outcomes of the EIN vs. EN group
To evaluate the relative impact of supplementing EIN with normal EN in GC patients, the postoperative clinical outcomes, including postoperative infections, postoperative complications, and postoperative systemic inflammation, were examined, as depicted in Figure 7. The EIN group had a lower likelihood of post-operative infections, with an OR of 0.63 (95% CI: 0.41–0.77) with a χ2 value of 7.42, df = 5, Z = 3.59, I2 = 33%, and p = 0.0003 (Figure 7 A). Similarly, the EIN group had a lower likelihood of post-operative complications, with an OR of 0.63 (95% CI: 0.44–0.90) with a χ2 value of 9.23, df = 6, Z = 2.57, I2 = 35%, and p = 0.01 (Figure 7 B). Furthermore, the likelihood of post-operative systemic inflammations was also low in the EIN group, with a SMD of –1.05 [95% CI: –1.62 to –0.49] with a τ2 value of 0.36, χ2 = 35.15, df = 4, Z = 3.68, I2 = 89%, and p = 0.0002 (Figure 7 C).
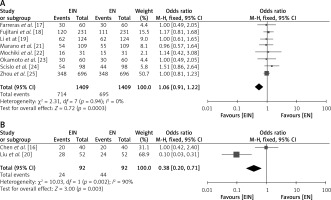
Effect of mode of supplementation of nutrients in the EIN vs. EN group
The relative impact of supplementing EIN with normal EN through either the oral or nasogastric mode in GC patients was examined, as depicted in Figure 8. The EIN group had a greater likelihood of improvement in serum protein levels, immunological factors, and post-operative issues with both the oral and nasogastric modes, as evident from an OR of 1.06 (95% CI: 0.91–1.22) with oral mode of feeding (χ2 value of 2.31, df = 7, Z = 0.72, I2 = 0%, and p = 0.0003) (Figure 8 A) as well as with nasogastric mode with an OR of 0.38 (95% CI: 0.20–0.71 with a χ2 value of 10.03, df = 1, Z = 3, I2 = 90%, and p = 0.003) (Figure 8 B).
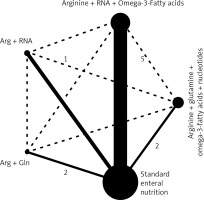
Evaluation of evidence networks among different components of enteral immunonutrition formulae
Figure 9 illustrates the evidence networks that were analysed with the objective of examining components of the various enteral immunonutrition formulas and their effects on immunological parameters, serum protein levels, and post-operative problems. The black solid line in the graph illustrates the direct comparisons made between regimes as conducted in the original research. Conversely, the black dashed line signifies the indirect comparisons made between two regimes that were not explicitly examined in the original research. The weights assigned to the nodes and edges were determined based on the overall sample size and standard error, respectively. The level of quality exhibited by the direct evidence varied from low to moderate. The potential of EIN was shown to be significant in improving serum protein levels and immunological parameters, while also lowering post-operative complications. These findings are backed by correct and clear connections throughout with high-quality data. This finding affirms that the provision of adequate dietary support, supplemented with immunonutrition, plays a significant role in facilitating the recovery of gastric cancer patients who have undergone gastrectomy.
Discussion
Gastric cancer is globally recognized as the fourth most frequent form of cancer and is the second leading cause of death [36]. Patients who have been diagnosed with gastric cancer often encounter nutritional deficiencies, which can worsen significantly following tumour removal surgery. Malnutrition commonly results in diminished cellular and humoral immune function, modifications in the inflammatory response, and a potential impediment or inability to properly heal wounds [37, 38]. Hence, in the context of patients undergoing postoperative care, the implementation of dietary aid measures has gained significant importance and prevalence [39, 40]. Both EN and EIN are components of nutritional treatment. However, EIN is often favoured due to its enhanced capacity to modulate various metabolic, inflammatory, and immunological processes [41, 42]. The European Society for Clinical Nutrition and Metabolism (ESPEN) also supports the utilization of early EIN in surgical patients afflicted with upper gastrointestinal malignancy to mitigate significant infection problems [43]. While several studies have demonstrated a decrease in post-operative complications and other favourable outcomes associated with EIN treatment [44, 45], the superiority of EIN over EN in terms of clinical and immunological indices remains a topic of debate.
The impact of EIN on patients with GC after surgical procedures was discussed in a meta-analysis by Song et al. in 2015 [46]. The results of the study revealed that the implementation of EIN had a beneficial impact on the nutritional and immunological well-being of GC patients who underwent surgical resection. In particular, it was shown that EIN led to an elevation in the concentrations of CD4+, CD4+/CD8+, CD3+, IgA, IgG, IgM, and NK cells. Nevertheless, the intervention did not provide a substantial influence on the levels of CD8+ cells, serum protein concentrations, surgical complications, or the duration of hospital stay. Similarly, in their meta-analysis, Fu et al. (2021) [47] also observed that endoscopic intranasal (EIN) administration, when compared to EN, resulted in a significant enhancement of immune and inflammatory factors as well as serum protein levels in patients with gastric cancer who underwent gastrectomy. However, no significant differences were found in terms of surgical wound infection or infectious complications. Several studies [48–50] have indicated that certain nutrients with immunomodulatory properties, such as ω-3-fatty acids, arginine, and dietary nucleotides of EIN, have the potential to support homeostasis after surgery and reduce inflammatory responses. These nutrients have been found to improve postoperative immune function and decrease post-operative complications following gastric surgery.
In our study, we applied a more extensive search strategy to investigate the effects of specific nutrient substances, including Arg, Gln, ω-3-FAs, and RNA, on the application of enhanced immunonutrition (EIN) compared to standard EN. Our findings indicate that EIN significantly enhances the post-operative levels of serum protein and immunological parameters, as shown by a significant standardized mean difference of 2.39 (95% CI: 0.13 to 4.66) for serum proalbumin, an SMD of 2.39 (95% CI: 0.13 to 4.66) for serum transferrin, an SMD of 1.34 (95% CI: 0.39 to 3.07) for lymphocyte count, and an SMD of 0.72 (95% CI: 0.13 to 3.31) for ratio of CD4+/CD8+. Additionally, EIN demonstrates a reduction in overall postoperative infectious complications with an OR of 0.63 (95% CI: 0.41–0.77) for post-operative infections, an OR of 0.63 (95% CI: 0.44-0.90) for post-operative complications, and an SMD of –1.05 (95% CI: –1.62 to –0.49) for post-operative systemic inflammations. All the observed outcomes exhibited statistical significance (p < 0.05), indicating a preference for the utilization of EIN in patients with gastric cancer who have gastrectomy, in comparison to EN. In their study, Gumusoglu et al. (2022) [51] presented findings indicating that timely identification of gastrointestinal system (GIS) anastomosis-related issues contributes to decreased mortality and morbidity rates. Additionally, the authors suggested that the supplementation of interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α), C-reactive protein (CRP), and procalcitonin (PCT) may serve as a viable approach for the early detection of significant issues in gastric cancer patients who are undergoing the Enhanced Recovery After Surgery (ERAS) protocol. Furthermore, it is recommended that imaging techniques be employed in individuals exhibiting elevated levels of each of these inflammatory chemicals on both the third and fifth postoperative days (POD). In a study conducted by Zhang et al. (2021) [52], a comparison was made between the outcomes of robot-assisted gastrectomy with D2 lymphadenectomy (RAGD2) and laparoscopy-assisted gastrectomy with D2 lymphadenectomy (LAGD2) in patients diagnosed with gastric cancer. The researchers discovered that while the RAGD2 procedure necessitated a lengthier duration in the operating room, it exhibited potential advantages in terms of lowering both intraoperative blood loss and postoperative problems when compared to the LAGD2 procedure.
Limitations
One significant aspect of this study is the utilization of extensive search phrases, which encompass specific immunonutrition components and multiple databases. Nonetheless, there are some limitations that should be mentioned. The main limitation of this analysis was the exclusion of studies conducted in languages other than English. Furthermore, it is important to consider the potential presence of selection bias in our study, because a considerable number of papers were eliminated from our meta-analysis. Additionally, we were unable to determine if the results were associated with gender, age, or ethnicity. In addition, this meta-analysis utilized a limited sample size consisting of 10 studies which exhibited notable variability, and considerable heterogeneity arose from different management programmes, dosages, health care organizations, length of EIN, and follow-up periods among the individuals.
Conclusions
The results of a meta-analysis indicate that EIN has a significant impact on various factors such as surgical site infections, infectious complications, systemic inflammatory response syndrome, and levels of CD8+, CD4+, CD4+/CD8+, lymphocytes, proalbumin, and transferrin in patients with gastric cancer undergoing a total gastrectomy as compared to EN. The findings suggest that EIN is more effective than EN in enhancing immune function in gastric cancer patients post-surgery. However, it is important to approach the analysis of the results with caution due to the limited number of studies included and the small sample sizes observed in many of these studies. Therefore, it is recommended that further research be conducted to validate these findings or potentially enhance the level of confidence in the assessment of the effects and to substantiate these conclusions.