Summary
Stroke of extracranial carotid artery origin (carotid-related stroke – CRS) occurs mostly with a thrombotic transformation of carotid atherosclerotic stenosis. Less frequent mechanisms include spontaneous or traumatic dissection or a large embolic load (usually from the heart) that may be superimposed on a pre-existing carotid stenosis. Intravenous thrombolysis is not only poorly effective (recanalization likelihood < 10%) but also it can enhance cerebral embolism with its carotid thrombus fragmentation. CRS is typically larger than intracranial large vessel occlusion stroke and it is often disabling. With its large thrombo-embolic load and “tandem” (extracranial carotid plus intracranial large-vessel) occlusions in about one in every 2 patients, CRS poses significant management challenges. While stenting (compared to non-stenting) generally improves outcomes, use of conventional (single-layer) stents is associated with a significant risk of new cerebral embolism and a significant risk of stent thrombosis. Single-layer stents are poorly optimized, in particular in CRS, to minimize the ‘cheese-grater’ effect. In consecutive CRS patients eligible for emergency recanalization we evaluated, in a multi-center multi-specialty investigator-initiated study, outcomes of using MicroNET-covered (cell area ≈ 0.02–0.03 mm2) carotid stent (CGuard, InspireMD) in otherwise routine clinical practice. Treatment, other than study device use (i.e., anticoagulation and antiplatelets doses and timing, use of other devices such as aspiration catheters vs. stentrievers, use of cerebral protection devices, sequence of intracranial intervention and stent placement in “tandem” lesions, etc.), was according to centre/operator routine (real-life, all-comer study). With protocol-recommended study stent post-dilatation optimization, the rate of technical success was high (100%) and clinical success was also high (82.7%) in the context of CRS outcomes with other stents and strategies. Heparin-limited-to-flush predicted patency loss on univariate but not on multivariate analysis. Small-diameter balloon/absent post-dilatation was an independent predictor of stent patency loss. Findings from this largest to-date multi-center evaluation of the MicroNET-covered stent use in consecutive patients with CRS subjected to endovascular stroke treatment may inform selection of the management strategy in acute ischemic stroke of carotid artery origin.
Introduction
One in every four ischemic strokes is linked to extracranial carotid artery stenosis or occlusion (extracranial carotid-related stroke – CRS) [1–3]. Although in some subjects carotid occlusion may occur in absence of symptoms, once an overt neurologic deficit develops the stroke is often large and disabling [3]. CRS natural history is associated with neurologic outcomes significantly worse than those seen with isolated intracranial large vessel occlusions (LVO) [3–7]. CRS, like other LVO strokes, is a cerebral emergency [3, 8], and non-delayed revascularization equals (cerebral) resuscitation [3, 8].
With the typical large thrombus burden of CRS, any effective recanalization using intravenous thrombolysis (IVT) is highly unlikely (recanalization rates < 10%) [3]. Also IVT use in CRS is associated with an increased risk of thrombus fragmentation and distal migration to a new cerebral territory, enhancing unfavorable clinical outcomes [9, 10]. Moreover, CRS-IVT increases the risk of bleeding and bleeding-related poor clinical outcomes [11, 12]. Thus, in CRS, the overall clinical value of IVT (particularly when IVT is not followed by endovascular stroke therapy (EST)) is questionable [3].
HERMES Collaboration-aggregated randomized controlled trials (RCTs) provided level-1 evidence that acute ischemic strokes caused by occlusion of the cerebral proximal anterior circulation, require – in cerebral-eligible patients – routine EST to be performed on an emergency basis, irrespective of whether any IVT use [13]. The therapeutic effect of EST is extremely powerful, with less than 3 patients needed to be treated to reduce one stroke-related disability [13]. Regrettably, the LVO-EST RCT protocols largely excluded tandem CRS [2], while in the studies that allowed tandem CRS patient enrollment the proportions of included patients were very low [13]. Even more importantly, no RCT has enrolled non-tandem CRS [8, 13] (i.e., carotid-only CRS, CRS in absence of concomitant intracranial LVO), a frequent CRS presentation [14]. The CRS under-representation in LVO-EST RCTs likely arises from significantly increased risks associated with the CRS revascularization procedure, including iatrogenic cerebral embolism (20–30% with conventional stent use) and challenges of addressing the carotid lesion (stent versus no stent, which stent, cerebral protection vs. no protection, associated pharmacotherapy, etc.) [2, 7, 9, 15, 16]. It is unrealistic to expect that HERMES-like trials would be repeated today for, specifically, CRS as a subtype of LVO ischemic stroke [3]. Evidence-based medicine integrates individual clinical expertise with the best available external clinical evidence [17]. In the case of absence of RCTs and meta-analyses with regard to a specific condition, evidence-based medicine decision-making is based on the best data available [17–19]. It is thus unethical to justify CRS-EST treatment denials with absence of CRS-specific RCTs [3, 20].
CRS emergency mechanical treatment has been effectively carried out for over 20 years, translating into improved clinical outcomes [3, 6, 18, 21–26]. Aggregated data indicate that stenting of the carotid lesion improves overall outcomes in CRS [16, 19, 27] but – with conventional stents – there is a clinically significant risk of stent thrombosis and occlusion. The 20–30% risk of new cerebral embolism with single-layer stents translates into their poor (or absent) postdilatation optimization [7, 25, 28, 29], while poor stent embedding, residual stenosis and too small stent diameter are well-known factors of stent patency loss [30]. Registry data indicate that the risk of stent occlusion with 1st generation stent use in CRS may exceed 30% [25].
The MicroNET-covered neuroprotective carotid stent, with its free-cell area of ≈0.02–0.03 mm2 [31, 32] insulates the atherothrombotic material [33] and has level-1 evidence for a profound reduction of cerebral embolism in elective carotid artery stenting (CAS) [34] as well as evidence for effective prevention of post-procedural cerebral embolism [34, 35]. However, little is known about the MicroNET-covered stent performance in emergency carotid lesion management in CRS, as published reports are limited to small single-center series [36] of mostly non-consecutive patients [36, 37].
Aim
This investigator-initiated study was performed to evaluate, in a multi-center multi-specialty setting, peri-procedural and 90-day clinical and duplex ultrasound outcomes of use of the MicroNET-covered carotid stent (CGuard, InspireMD) – in the context of otherwise routine procedural and clinical management – in consecutive patients presenting with acute stroke in mechanistic relation to extracranial carotid artery stenosis or occlusion.
Material and methods
Consecutive CRS patients eligible for mechanical reperfusion therapy [3, 8, 18, 22, 24, 38] were enrolled in 7 interventional stroke centers, including four Comprehensive (level-3) Stroke Centers and three Thrombectomy-Capable (level-2) Stroke Centers. Enrollment occurred over a 12-month period; individual centers’ recruitment ranged from 3 to 12 months.
Acute ischemic stroke definition was according to current scientific statements [8]. Patients with carotid-related crescendo transient ischemic attacks (TIA), and stroke-in-evolution or stuttering stroke were also considered eligible for enrollment, consistent with prior studies [3, 39]. Crescendo TIA was defined as repetitive TIA episodes of increasing intensity over hours or days followed by return to apparently normal neurologic status [39]. Stroke-in-evolution was defined as progression of a neurologic deficit that occurred over ≥ 24 h without restoration of apparently normal neurologic status [39]. Fluctuating (“stuttering”) stroke was characterized by clearly separated episodes of progressive neurologic deficit without restoration of apparently normal neurologic status between episodes over at least a 24-hour period (within the ≥ 24-hour period the deficit could wax and wane but never clear completely) [39].
Guideline-indicated time and cerebral tissue windows [8] were protocol-recommended to be followed if applicable; otherwise management was per local standards and team expertise. Vascular access site and, in total occlusions, the technique employed to pass the extracranial carotid artery occlusion [15, 40–43] were left to the discretion of the operator.
Since (1) stent under-expansion and malapposition are well-known contributors to the risk of thrombosis, (2) residual stenosis is an important factor of in-stent restenosis, and (3) MicroNET-covered stent optimization has been shown to be safe and effective in symptomatic patients in elective CAS [31, 33], the study device post-dilatation was encouraged to optimize the implant embedding. However, post-dilatation balloon use, along with the balloon diameter and peak pressure, was left to the operator’s decision. In relevance to real-life clinical practice variability in CRS-EST [3, 21, 44–48], use of other devices (such as cerebral protection and its type, stentriever versus aspiration, sequence of recanalization and stenting in tandem lesions – anterograde vs. retrograde, etc.) and the type, timing and administration route of medications in relation to intervention (heparin, antiplatelets, others) were left to the operator discretion as per study center routine management pathway(s) and local expertise. Rather than forcing a protocol for highly selected patients, poorly corresponding to routine clinical practice [49], we intended to evaluate the MicroNET-covered stent performance in a real-life setting, relevant to all-comer CRS patients.
Procedural angiograms were submitted for assessment by an angiographic core lab technologist who evaluated the carotid culprit lesion type (atherosclerosis, dissection, proximal thromboembolism), angiographic presence of thrombus and calcifications [50], and final modified Thrombolysis in Cerebral Infarction (mTICI) flow [8].
Technical success was defined as the study device delivery and implantation with withdrawal of the delivery system and residual stenosis < 30% on procedure completion. Clinical success was defined as technical success in absence of clinical adverse events (death, myocardial infarction, clinically relevant stroke extension, symptomatic intracranial hemorrhage – sICH) or loss of the target internal carotid artery (ICA) patency by discharge.
The study was registered with respective Ethic Committees as per local regulations and was concordant with the Declaration of Helsinki, and patients provided informed written consent to participate. The study record was registered in a public database (ClinicalTrials.gov NCT05195658 SAFEGUARD-STROKE – Acute Stroke of CArotid Artery Bifurcation Origin Treated With Use oF the MicroNET-covered CGUARD Stent).
Statistical analysis
Continuous variables are reported as median (Q1–Q3) unless specified otherwise; categorical data are expressed as numbers and proportions. The Mann-Whitney U test or Wilcoxon matched pairs test was used for comparisons. Bonferroni correction was applied for multiple comparisons. To determine predictors of symptomatic intracranial hemorrhage (sICH), unfavorable clinical outcome (modified Rankin Scale [mRS] > 2) and study stent patency loss, univariate and multivariate models including potential clinical and imaging variables were constructed. Statistical significance was defined as p < 0.05. SPSS v. 29 (IBM Corporation, New York, United States) was used for computations.
Results
Seventy-five consecutive CRS patients (age 40–89 years, median: 67 years, 26.7% women) were enrolled. Baseline characteristics of study subjects are provided in Table I. Median Alberta Stroke Program Early CT Score (ASPECTS) was 9 (range: 6–10). Twenty-nine (38.7%) patients had received IVT. A great majority had hyperacute stroke presentation (86.7%). TIA preceded the index CRS in 18.7%; 8% of patients presented with crescendo TIA or stroke-in-evolution. Stuttering/aggravating stroke symptoms were present in 4 study subjects (5.3%), including 3 patients accepted for treatment within the study with mRS 3 or 4 in the context of progressive stroke aggravation. The majority were tandem strokes (extracranial internal carotid artery acute occlusion non-tandem stroke in 48%). Atherosclerotic stenosis dominated as the underlying mechanism (89.3%; dissection – 6.6%, embolic load from proximal circulation superimposed on carotid stenosis – 3.9%).
Table I
Baseline characteristics (n = 75)
| Parameter | Value |
|---|---|
| Age [years]: | 67 (61–74) |
| Range | 40–89 |
| Female gender | 21 (28.0) |
| ASPECTS on admission: | 9 (8–10) |
| Range | 6–10 |
| NIHSS on admission: | 14 (12–19) |
| Range | 6–27 |
| mRS prior to index stroke onset: | 0 (0–1) |
| Range | 0–3 |
| Time from symptom onset to presentation [h] | 5 (3–11) |
| Range | 1–38 |
| IVT | 29 (38.7) |
| Hypertension | 67 (89.3) |
| Coronary artery disease | 26 (34.4) |
| Atrial fibrillation | 10 (13.3) |
| TIA preceding index stroke | 14 (18.7) |
| Stroke in history | 7 (9.3) |
| Symptomatic PAD | 8 (10.7) |
| Diabetes: | |
| Type 1 | 1 (1.3) |
| Type 2 | 19 (25.3) |
| Prediabetes | 6 (8.0) |
| Smoker: | 41 (54.7) |
| Current | 25 (33.3) |
| Ex-smoker | 16 (21.3) |
| Hypercholesterolemia or hypolipidemic therapy prior to stroke | 62 (82.7) |
| History of neck/chest radiotherapy | 3 (4.0) |
| Type of stroke (clinical): | |
| Hyperacute | 65 (86.7) |
| Stroke-in-evolution or crescendo TIA | 6 (8.0) |
| Stuttering/aggravating | 4 (5.3) |
| Stroke side, left | 39 (52.0) |
| Lesion/occlusion level(s): | |
| Tandem (extra-plus intracranial) | 39 (52.0) |
| Isolated extracranial | 36 (48.0) |
| ICA lesion type: | |
| Atherosclerosis | 67 (89.3) |
| Dissection | 5 (6.6) |
| Thrombo-embolic load from prox circulation* | 2 (2.3) |
| ICA thrombus† | 47 (62.7) |
| ICA highly calcific stenosis‡ | 11 (14.7) |
Values are given as median (Q1-Q3) or n (%) as applicable. ASPECTS – Alberta Stroke Program Early CT Score.
#evidence of cerebral vessel occlusion on CTA or cQA (embolic carotid-related stroke mechanism was considered applicable in case of a non-flow limiting carotid lesion),
‡ calcific segment length to lesion length ≥ 2/3, minimal calcification thickness ≥ 3 mm, circularity (≥ 3 quadrants), and calcification severity grade ≥ 3 [50].
Key procedural data are given in Table II; typical clinical and imaging scenarios are provided in raw data figures (Figures 1–6). Cerebral protection devices were used in less than half of study procedures (45.3%), with a clear dominance of proximal systems (Table II). Thrombus aspiration was the first-line strategy in the majority of extracranial and intracranial occlusions. Intracranial and large-bore carotid stentrievers were used mostly in conjunction with aspiration in the case of failure of the aspiration-alone strategy. Nevertheless, some operators preferred to start with stentriever extraction as a preferred strategy (Table II).
Table II
Procedural data (n = 75)
| Parameter | Value |
|---|---|
| Access site: | |
| Femoral | 67 (89.3) |
| Radial | 5 (6.7) |
| Transcarotid± | 3 (4.0) |
| Anaesthesia | |
| General | 41 (54.7) |
| Conscious sedation | 34 (45.3) |
| Cerebral protection device: | 34 (45.3) |
| Proximal: | 29 (38.7) |
| MoMa system | 19 (25.3) |
| Mono-balloon catheter | 8 (10.7) |
| TCAR | 2 (2.7) |
| Distal (filter) | 3 (4.0) |
| Double (Mono-balloon catheter + filter) | 2 (2.7) |
| No protection device | 41 (54.7) |
| Thrombus extraction* | |
| In n = 47 extracranial thrombotic lesions: | 45 (95.7) |
| Aspiration-only | 42 (89.4) |
| Large-bore ST (under aspiration) | 3 (6.4) |
| In n = 39 intracranial LVOs: | 37 (94.9) |
| Aspiration-only | 19 (48.7) |
| Aspiration followed by ST | 15 (38.4) |
| ST as primary strategy | 3 (7.7) |
| Intracranial MT (n = 37): | |
| ICA | 2 (5.4) |
| ACA | 1 (2.7) |
| M1 | 21 (56.8) |
| M2 | 5 (13.5) |
| Multisite intracranial | 8 (21.6) |
| Number of passages in intracranial MT | 2 (1–4) |
| 1–9 | |
| Primary (‘direct’) stenting | 29 (38.7) |
| Extracranial lesion predilatation | 46 (61.3) |
| Predilation balloon diameter [mm] | 3.5 (3.0–3.5) |
| Range | 1.0–5.0 |
| Carotid stent strategy in tandem lesions: | |
| Antegrade | 12 (30.8) |
| Retrograde* | 27 (69.2) |
| Total number of study stents used | 78 |
| Non-study stent use | 0 |
| Stent size, diameter [mm] × length [mm]: | |
| 6 × 40 | 2 (2.6) |
| 7 × 30 | 5 (6.4) |
| 7 × 40 | 5 (6.4) |
| 8 × 30 | 9 (11.5) |
| 8 × 40 | 7 (9.0) |
| 9 × 30 | 12 (15.3) |
| 9 × 40 | 16 (20.5) |
| 10 × 30 | 6 (7.7) |
| 10 × 40 | 10 (12.8) |
| 10 × 60 | 4 (5.1) |
| > 1 stent implantation, n (% culprit ICA): | 3 (4.0) |
| Second stent reason: | |
| Dissection | 0 |
| Thrombus | 0 |
| Lesion length# | 3 (4.0) |
| Post-dilatation performed | 72 (96) |
| Postdilation balloon peak diameter [mm] | 5.0 (5.0–5.5) |
| Range | 4.0–8.0 |
| Postdilation balloon peak pressure [mm Hg] | 18 (12–20) |
| Range | 8–24 |
| New cerebral embolism with stent delivery/implantation† | 1 (1.3) |
| Final mTICI, n (%): | |
| 0/1 | 3 (4.0) |
| 2a | 5 (6.7) |
| 2b/c | 17 (22.7) |
| 3 | 50 (66.7) |
| Procedure duration [min]: | 70 (45–97) |
| Range | 33–170 |
| Intraprocedural heparin use | 75 (100) |
| Intraprocedural heparin regimen: | |
| Catheter(s) flush only | 6 (8.0) |
| Additional dose(s): | 69 (92.0) |
| 1500–3000 IU | 11 (14.7) |
| 3000–5000 IU | 21 (28.0) |
| ACT-adjusted dosing with ≥ 250 s target | 37 (49.3) |
| Peri-procedural antiplatelet administered (at least 1): | 69 (92.0) |
| iv ASA | 7 (9.3) |
| Oral/nas-gastric tube ASA | 59 (78.7) |
| GP IIb/IIIa inhibitor: | 16 (21.3) |
| IA bolus only | 4 (5.3) |
| IA bolus + IV infusion | 12 (16.0) |
| Cangrelor | 3 (4.3%) |
| Post-procedural antiplatelet(s): | 75 (100) |
| One (ASA or clopidogrel) | 4 (5.3) |
| Two (ASA plus clopidogrel) | 71 (94.7) |
| Timing of second antiplatelet administration (n = 71) | |
| ≤ 24 h | 38 (53.5) |
| > 24 h | 22 (46.5) |
| Delay [h] | 28 (26–31) |
| Delay, range | 24–48 |
| Recommended DAPT duration [months] | 3 (3–3) |
| range | 1–12 |
Figure 1
MicroNET-covered stent management of athero-thrombotic carotid-related non-tandem wake-up stroke. A 68-year-old woman with chronic occlusion of the left internal carotid artery (that had previously manifested as a TIA) was admitted to a thrombectomy-capable stroke center with a wake-up stroke (National Institutes of Health Stroke Scale (NIHSS) 17). Cerebral plain computed tomography (CT) demonstrated Alberta Stroke Program Early CT Score (ASPECTS) 9. Magnetic resonance cerebral imaging showed chronic bilateral lesions (A, white arrows) with diffusion-weighted imaging acute focal lesions located mostly in the right but also in the left hemisphere (A, yellow arrows; the lesions were absent on fluid-attenuated inversion recovery imaging). Computed tomography angiography (CTA) demonstrated an occlusion of the left internal carotid artery (LICA) and a near-occlusion of the right internal carotid artery (RICA) by a thrombotic/soft lesion. Intra-arterial angiography (femoral access) demonstrated a tight, highly thrombotic (red arrowheads) lesion in the right carotid artery bifurcation (B, note that duplex imaging 11 months earlier had shown ~50–60% stenosis that, according to guidelines, had been managed with maximized medical therapy including low-dose aspirin, angiotensin-converting enzyme inhibitor (ACEI), and a high-dose statin titrated to achieving LDL-cholesterol level < 70 mg/dl). There was absence of intracranial arterial occlusions; a slow-flow supply visualized only the right cerebral anterior circulation vessels (C). Proximal cerebral protection was established with a balloon catheter (white arrows, D–G, ‘back’ pressure 42/37 mm Hg), enabling a passive transient flow reversal throughout the procedure. Dotted arrows in D-G indicate LICA/left common carotid artery (LCCA) reversed flow direction. After a small-balloon (2.0 × 20 mm) predilation (E) a MicroNET-covered CGuard 9 × 30 mm stent was implanted to sequestrate the athero-thrombotic material (F). The stent was optimized with 5 × 20 mm balloon at 18 atm (G). Active aspiration was performed despite the (increasing) clinical intolerance manifested by transient aggravation of symptoms; the total flow-reversal time was 6 min and 40 s. The thrombotic material aspirated during the procedure is shown in H–I (inset). H is the stent image; note the full correspondence to the anatomy with its self-tapering (‘SmartFit’ feature). I is the final angiographic result of the stroke culprit lesion revascularization; note the optimal endovascular reconstruction of the carotid bifurcation with absence of any in-stent material and external carotid artery branches (normal opacification). Final cerebral angiogram (J) showed resumption, as a result of the procedure, of not only a normal modified Thrombolysis in Cerebral Infarction scale (mTICI) 3 flow to the right cerebral hemisphere but also with a rich supply to the left hemispheric vessels. There was a nearly immediate symptom resolution, with almost complete normalization by the next day. Ninety-day duplex ultrasound follow-up (K) showed a patent stent with laminar flow, absence of any in-stent material, and slightly increased in-stent velocities (1.4/0.65 m/s; consistent with flow compensation of the contralateral occlusion). White arrows in K show hypoechogenic material sequestrated by the stent. Control cerebral CT (L) demonstrated only minor vasculogenic lesions while the neurologic outcome was excellent (mRS 1)
B/L – baseline, CTO – chronic total occlusion; mRS – modified Rankin score; 90d – 90 day.
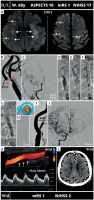
Figure 2
MicroNET-covered stent ‘antegrade strategy’ management of carotid-related thrombotic tandem-lesion acute stroke resulting from a spontaneous dissection of extracranial carotid artery atherosclerotic lesion. A 61-year-old left-handed man was admitted to a comprehensive stroke center 50 min after right hemispheric stroke onset (NIHSS 19). Plain CT showed hyperdense right middle cerebral artery (RMCA) “dot sign” (red arrowhead, A) in absence of CT early stroke changes (ASPECTS 10, A). CTA (not shown) demonstrated a tandem-lesion occlusion of the right cerebral anterior circulation vessels (extracranial ostial RICA occlusion and co-existing occlusion of 2 RMCA branches). This patient, with a family history of stroke, had been diagnosed a year earlier with a ‘non-significant’ RICA stenosis and was put on a low-dose aspirin and a high-dose statin. Baseline carotid angiography (femoral access) confirmed ostial RICA occlusion (B, RICA stump, red arrow). The occlusion was crossed with a microwire and microcatheter (contrast injection via microcatheter was performed to confirm true lumen cannulation). A MicroNET-covered antiembolic CGuard 10 × 60 mm stent (the longest available length) was implanted directly in the dissected segment (C). Post-implantation angiography (C) showed persistent occlusion at the proximal stent edge, indicating that the very proximal dissection segment remained uncovered (red arrowheads in C). After proximal implantation of a second CGuard stent (10 × 40 mm; overlapping stent technique) and postdilatation optimization of the two stents (5 × 20 mm balloon inflations distally, proximal inflation with a 7 × 20 mm balloon at 14 atm), the extracranial carotid lumen was effectively reconstructed (D and E; white arrows indicate edges of the top stent while blue arrows show the bottom stent edges; the segment between the blue and white arrow in the middle of D and E is the segment of overlapping stents; absence of flow to right external carotid artery (RECA) via the reconstructed lumen suggests recanalization performed via, in part, the false lumen). The intracranial lesions were then addressed (“bottom-to-top” strategy, a.k.a. “antegrade” strategy). Intra-arterial angiography confirmed occlusion of two M2 branches of the RMCA (F, red arrowheads). Recanalization was achieved by using a combined stentriever (Trevo 4 × 20 mm; G) and Sofia 6F aspiration catheter technique. One of the occluded RMCA branches was effectively opened (evacuated thrombus in H), whereas several attempts to open the other occluded branch were unsuccessful (I; red arrowhead denotes a remaining occlusion). Final mTICI flow was 2a (J). On clinical follow-up at 90 days, mRS was 2 and NIHSS was 5, consistent with a good clinical outcome; duplex scan demonstrated patent stents with normal flow. CTA follow-up at 6 months confirmed patent overlapping stents without any in-stent material (K). Despite a significant cerebral tissue loss in the right hemisphere (L) the clinical outcome was good (mRS 2, functional independence).
postdil – post-dilatation, RCCA – right common carotid artery, 180d – 180 days.
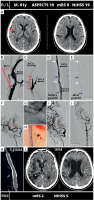
Figure 3
MicroNET-covered stent management of athero-thrombotic extracranial carotid-related non-tandem acute stroke in a patient with lack of femoral arterial access and an ineffective transradial access. A 59-year old woman was admitted with a wake-up right hemispheric stroke (NIHSS of 14). Plain CT ASPECTS was 9. CTA imaging of the aortic arch and intracranial vessels did not demonstrate an intracranial occlusion. The RCCA was disease-free but there was a thrombotic near-occlusion in the proximal RICA. Magnetic resonance imaging (MRI) (A) showed an acute ischemic lesion in the right ‘corona radiata’ region (note hyper-intense signal lesions on diffusion-weighted imaging (DWI) that are dark on apparent diffusion coefficient (ADC) maps and are invisible on fluid-attenuated inversion recovery (FLAIR) sequence (yellow arrows) – DWI/FLAIR “miss-match”; consistent with an early hyperacute stroke amenable to emergent revascularization). There was absence of femoral arterial access (Leriche syndrome). Right radial access failed due to an extremely sharp angle between the subclavian artery and common carotid artery. A “direct” common carotid artery access was established (TCAR technique [59], blue arrow in B). Baseline angiography using a 5F radial sheath (B) demonstrated a tight, highly thrombotic (red arrowheads in B) lesion in the proximal RICA (white arrow in B shows the point of radial sheath entry into the RCCA, black double-arrow denotes the tip of the 5F sheath). The 5F sheath was then exchanged for the 8F EnRoute dynamic flow reversal system [59]. Antegrade angiography confirmed a highly thrombotic RICA lesion (C) with severely impaired supply to the right hemisphere (consistent with the DWI ischemia in A) in absence of intracranial vessel(s) occlusion (D). With the TCAR dynamic flow reversal (E, ‘retrograde’ angiography with backflow of non-contrasted blood via both RICA and RECA), note partial disappearance of the thrombotic material in the RICA (visible in B and C but replaced with a column of contrast in E, green arrowheads, consistent with evacuation of part of the thrombi with the dynamic flow reversal; dotted arrows indicate flow direction). Following a small-diameter (2.5 × 20 mm) balloon inflation to further enhance the reversed flow cerebral protection, a 8.0 × 30 mm antiembolic CGuard stent was inserted, and it was post-dilatation embedded with a 5 × 20 mm balloon at 16 atm (F). The stent image is presented in G (note the fit to the anatomy) whereas H is the final angiogram (note the full patency of the RECA branches). The inset in H is a photograph of the EnRoute system external filter used in this procedure (note numerous thromboembolic pieces that were effectively evacuated). The final cerebral angiogram (I) showed a normal (mTICI-3) flow to the right hemisphere; note absence of intracranial embolism that was effectively prevented using a combination of flow reversal and the MicroNET-covered stent. Ninety-day follow-up showed a patent stent with absence of any in-stent material and normal-velocity laminar flow (J). Control MRI showed limited permanent ischemic lesions (arrows, K). The functional outcome was excellent (mRS 1)
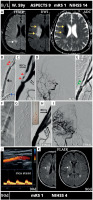
Figure 4
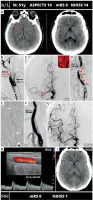
MicroNET-covered stent management of carotid-related tandem-lesion acute stroke resulting from extracranial carotid artery thrombosis on the basis of a highly calcific lesion. A 73-year-old man with chronic CAD and a chronic LCCA/LICA occlusion was admitted to a level-3 (i.e., thrombolysis-capable) stroke center 80 min after right hemispheric stroke onset (admission NIHSS was 22). Plain CT showed a hyperdense middle cerebral artery (MCA) sign on the right (A, red arrowhead) with ASPECTS 10. CTA demonstrated an occlusion of the RICA (red arrow, B) on a massively calcific lesion (yellow arrowheads, B). Cerebral CTA demonstrated RMCA thrombotic occlusion (C, red arrowhead), consistent with the hyperdense MCA sign in A. Intravenous thrombolytic therapy was immediately initiated. Two hours later, in absence of a clinical improvement (NIHSS 20) the patient was transferred to a level-2 (thrombectomy-capable) stroke center. Cerebral CT was repeated (D) to exclude cerebral bleed and evaluate the cerebral parenchyma; ASPECTS was 9. Carotid catheter angiography (femoral access) confirmed a massively calcific lesion in the RCCA bifurcation (E, yellow arrowheads indicate the ‘stone of calcium’; note a dotted line around the calcium conglomerate) and proximal RICA near-occlusion (non-subtracted image in E, subtracted image in F). A highly angulated narrow flow canal was visible inside the calcium conglomerate; sluggish antegrade flow was consistent with an effect of thrombolysis (recanalized RICA in E–F, compare absence of antegrade flow in B). Intracranial angiogram (G) showed limited, sluggish flow to the right hemispheric cerebral vessels but no longer evidence of intracranial occlusion (thrombolysis partly effective). Severe calcifications prohibited insertion of a double-balloon catheter (note that the ECA balloon did not cross the distal RCCA heavy calcifications); this was replaced with a mono-balloon catheter (reversed flow direction is denoted by a dotted arrow in H to K). Attempts to insert a Ø2.0 mm or Ø1.5 mm balloon through the lesion failed, and predilatation (I) was thus started with a Ø1.0 mm CTO-dedicated coronary balloon (I-1). This was followed by use of a Ø2.0 mm and Ø3.0 mm Scoring Balloon (SB, I-2); then Ø3.5 mm and Ø4.0 mm cutting-balloon inflations were performed (CB, I-3). Final lesion preparation involved Ø4.0-5.5 NC balloons at high pressures (I-4). Then, to minimize the risk of perforation and post-stenting embolism, a dual-layer MicroNET-covered CGuard 10 ×40 mm stent was used (the stent was inserted under continued flow reversal, J – note a continually inflated low-pressure balloon of the balloon catheter, white arrow). The anti-embolic stent was then optimized with Ø6.0 mm non-compliant (high-pressure) balloon (NC balloon) sequential inflations up to 24 atm (K). This led to a fully reconstructed RCCA bifurcation, with a normal lumen restoration in RCCA/ RICA and a normal RECA flow (L). The total flow reversal time was 28 min. Reversed flow, ensuring cerebral protection against plaque-related embolism, was intentionally maintained throughout the procedure despite increasing intolerance. The final cerebral angiogram (M) showed a normal (mTICI-3) flow to the right hemisphere vessels in absence of any distal embolism and a vivid intracranial collateral supply to the left hemispheric vessels; inset in M shows the scoring balloon (SB) and cutting balloon (CB) that permitted this very highly calcific stroke culprit lesion effective predilatation, and enabled (together with a dual-layer stent) fully endovascular management. Note that combining surgical endarterectomy with intracranial mechanical thrombectomy may not be optimal [3], while the MicroNET-covered stent can be effectively used in intra-arterial treatment of highly calcific carotid bifurcation lesions [50]. Ninety-day follow-up showed a patent stent with laminar flow and normal velocities (N). Control CT showed only limited permanent ischemic lesions in the basal ganglia on the right (O, white arrows). Clinical outcome was excellent (mRS 1)
occl – occlusion.
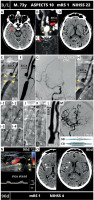
Figure 5
MicroNET-covered stent use to treat a stuttering carotid-related athero-thrombotic non-tandem stroke. A 53-year-old woman was first admitted to a local hospital with TIA manifesting as a recurring transient blurred vision. Initial cerebral plain CT (A) was normal but on the next the symptoms progressed to a minor stroke (NIHSS 4). During continued hospitalization in a neurology ward, on day 5 the patient developed right eye vision loss with right hemiparesis and total aphasia. Repeated CT showed an infarct area in the left occipital lobe (B) in the left main coronary artery (LMCA) territory (ASPECTS 9). CTA demonstrated highly thrombotic LICA stenosis. Following a telephone consultation in a thrombectomy-capable stroke center with extensive CAS experience, the patient was put on heparin anticoagulation in addition to the antiplatelet treatment and was transferred to the revascularization center (level-2 stroke center). Baseline angiography (femoral access) demonstrated a highly thrombotic lesion with a mobile thrombus in the LICA (C and D, red arrowheads) and impaired flow to the left hemispheric vessels in absence of a cerebral vessel occlusion (E). Proximal protection was established with a mono-balloon catheter (white arrows in D and F-H); this enabled flow reversal throughout the procedure (dotted arrows indicate reversed flow direction). A MicroNET-covered antiembolic CGuard 10 × 40 mm stent was implanted directly in the LICA/LCCA (F, G) under the flow-reversal cerebral protection, excluding the thrombus from the lumen. Under continued flow reversal the stent was post-dilatation optimized with a 6 × 20 mm balloon (H) at up to 20 atm. Optimal reconstruction of the carotid bifurcation was achieved (I and J; note the stent full adaptation to the internal carotid artery/common carotid artery (ICA/CCA) anatomy in I, ‘SmartFit’ characteristics, and non-compromised patency of the external carotid artery (ECA) branches). Final cerebral angiogram (K) demonstrated normal (mTICI-3) flow to the left hemispheric vessels in absence of any embolism. Ninety-day follow-up showed patent stent with laminar flow and normal velocities (L). CT follow-up was performed at 6 months (M), demonstrating absence of any new ischemic changes relative to the preprocedural imaging in B (in M note a glial scar in the left occipital lobe, white arrows)
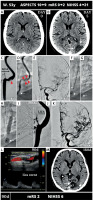
Figure 6
MicroNET-covered stent ‘retrograde strategy’ in managing carotid-related thrombotic tandem-lesion stroke resulting from extracranial carotid artery dissection with a large thrombus burden. A 51-year-old man was admitted to an interventional radiology-run stroke center in the 2nd hour of right hemispheric stroke onset. Plain CT showed a right hyperdense MCA sign (left image in A, red arrowhead); ASPECTS was 10 (A, image on the right. CTA (not shown) demonstrated a RICA-RMCA ‘tandem’ occlusion (totally occluded RICA, totally occluded and RMCA-M1). Baseline carotid angiography (femoral access) demonstrated RICA proximal occlusion (B); this was crossed using a microwire and a microcatheter set. Then a reperfusion catheter (JET 7) was inserted and ICA aspiration was performed, yielding a volume of thrombi. Intracranial angiography (C) confirmed RMCA thrombotic occlusion in the M1 segment (C, red arrowhead). This was treated by JET 7 aspiration (first pass recanalization effect). Gentle injection through a guide catheter showed a long proximal ICA dissection (D); an intra-arterial bolus of integrelin was administered, followed by an IV infusion. The dissection culprit lesion was resolved with a 6 × 40 mm MicroNET-covered stent (direct stenting). The stent was then optimized with a 5.5 × 20 mm balloon (inflations at 10–14 atm); there was an optimal angiographic result with no detectable cerebral embolism. Full reconstruction of the ICA lumen was achieved (E and F; the stent edges are indicated by white arrows; note a mild spasm at both edges of the stent, blue arrows; this resolved spontaneously). The final cerebral angiogram (G) demonstrated a normal (mTICI-3) flow to the right cerebral hemisphere with no detectable distal embolization. Ninety-day follow-up showed a patent stent with laminar flow and normal velocities (H) whereas control CT (I) demonstrated a normal brain
The primary (“direct”) stenting rate was 38.7%; the majority of lesions (61.3%) were predilated prior to stent placement. The retrograde carotid lesion stenting strategy (i.e., intracranial recanalization followed by stenting; stent placement “on the way down”) clearly dominated in tandem lesions (69.2%). Table II displays the study device matrix as per specific stent diameters and lengths used. The 9 × 40 mm study stent was selected most frequently (20.5%), followed by 9 × 30 mm, 10 × 40 mm and 8 × 30 mm. Lengths of 30 and 40 mm were used nearly equally, whereas the 60 mm length constituted a minority (5.1%) of operator-selected stent lengths. In a great majority of cases (96%) the study device was post-dilated. Postdilatation balloon diameter ranged from 4.0 to 8.0 mm (median 5.0 mm; Q1-Q3 5.0–5.5 mm); post-dilatation peak pressure ranged from 8 atm (nominal) to high pressures (“full optimization”) [30, 31]. Technical success rate was 100%. Acute clinical success rate was 82.7% (7 in-hospital deaths, 3 study device occlusions not related to death, 3 sICH not leading to death).
Intraprocedural unfractionated heparin was used in all cases (100%). In 8% of study patients heparin use was limited to catheter flush only. The other end of the heparin dose spectrum (Table II) reflected a strategy of activated clotting time (ACT > 250 s)-adjusted heparinization consistent with the study device instructions for use (every second study procedure, Table II). Ninety-two percent of study patients received peri-procedurally at least 1 antiplatelet agent (Table II); cangrelor use was limited to 3 (4.3%) cases. Glycoprotein (GP) IIb/IIIa inhibitor was used in 1 in every 5 patients, in the majority of cases as an intraarterial (IA) bolus + IV infusion. All study patients were on at least 1 antiplatelet agent post-procedurally. The 2nd antiplatelet agent administration was delayed by > 24 h in nearly 50% of subjects (46.5%). Final mTICI 2b/c-3 was achieved in a great majority of cases (89.4%).
Closure devices were used in 65.8% of procedures other than the surgical access site closure used in 3 cases employing the transcarotid approach. The main clinical and imaging outcomes of interest are presented in Table III. Seven (9.3%) patients died in hospital. Symptomatic intracranial hemorrhage occurred in 4 (5.3%). NIH Stroke Score (NIHSS) on discharge was greatly reduced compared with that on admission (from a median of 14 to 4, p < 0.01). The study stent patency rate at discharge (incorporating occlusions prior to death) was 94.3%. No in-stent material was present in the stents patent at discharge, and in-stent velocities were normal (Table III). By 90 days 1 (1.5%) patient experienced a new ipsilateral stroke (study device normally healed, embolic stroke in relation to de novo atrial fibrillation) and 1 (1.5%) other patient had a posterior circulation stroke. Ninety-day patency of the study device was 92.2%, with normal in-stent velocities and absence of in-stent material (Table III).
Table III
Main outcomes of interest
| Parameter | Results |
|---|---|
| In-hospital/by discharge (n = 75) | |
| Any intracranial hemorrhage: | 12 (16) |
| sICH | 4 (5.3) |
| asICH | 8 (10.7) |
| In-hospital death | 7 (9.3) |
| NIHSS# on discharge: | 4 (2–8) |
| Range | 0–23 |
| mRS at discharge: | 1 (1–3) |
| Range | 0–6 |
| Stent patent# on discharge | 66 (94.3) |
| DUS in-stent velocities: | |
| PSV [cm/s] | 69 (53–91) |
| EDV [cm/s] | 20 (12–26) |
| Any in-stent material | 0 (0) |
| 90-day outcomes† (n = 66) | |
| New stroke by 90 days, any: | 2 (3) |
| Ipsilateral | 1 (1.5) |
| Contralateral | 0 |
| Posterior circulation | 1 (1.5) |
| 90-day death (total*) | 9 (12.0) |
| NIHSS at 90 days | 3 (0–5) |
| mRS‡ at 90 days | 1 (1–2) |
| Stent patent¥ by 90 days | 59 (92.2) |
| Any in-stent material§ | 0 (0) |
| Stent occluded by 90 days | 5 (7.8) |
| DUS in-stent velocities: | |
| PSV [cm/s] | 64 (55–84) |
| EDV [cm/s] | 24 (21–30) |
Uni- and multivariate analysis explored predictors of fundamental outcomes of interest, including sICH (Table IV), bad clinical outcome (Table V), and stent patency loss by 90 days (Table VI). Glycoprotein (GP) IIb/IIIa inhibitor use as an IA bolus plus intravenous (IV) infusion, IVT, tandem lesion, carotid T-occlusion, and additional (defined as other than in flush) heparin dose were univariate predictors of sICH; however, GP IIb/IIIa inhibitor use as an IA bolus plus IV infusion remained as a sole sICH predictor on multivariate analysis. Large neurologic deficit (NIHSS > 20) and significant cerebral tissue loss (ASPECTS < 8) at baseline as well as GP IIb/IIIa inhibitor use as an IA bolus plus IV infusion independently predicted absence of functional independence at 90 days. Study device post-dilatation with balloon < 5 mm diameter (including absence of post-dilatation, Table II) was the sole independent predictor of stent patency loss by 90 days.
Table IV
Predictors of sICH
| Univariate | Multivariate |
|---|---|
| GP IIb/IIIa inhibitor IA bolus + IV infusion OR = 6.4 (1.8–24.5), p < 0.001 | GP IIb/IIIa inhibitor IA bolus + IV infusion OR = 16.9 (4.8–44.3), p < 0.001 |
| T-occlusion OR = 3.9 (1.9–15.1), p < 0.001 | |
| Tandem lesion OR = 3.4 (1.3–35.9), p = 0.010 | |
| IVT OR = 1.9 (1.1–20.6), p < 0.001 | |
| Additional dose of heparin# OR = 1.4 (1.1–18.7), p = 0.020 |
Table V
Predictors of bad clinical outcome (mRS > 2) at 90 days
| Univariate | Multivariate |
|---|---|
| GP IIb/IIIa inhibitor IA bolus + IV infusion OR = 23.8 (5.3–94.5), p < 0.001 | NIHSS > 20 OR = 14.7 (2.1–78.2), p = 0.006 |
| ASPECT < 8 OR = 11.2 (3.2–38.9), p < 0.001 | GP IIb/IIIa inhibitor IA bolus + IV infusion OR = 13.9 (5.1–84.5), p < 0.001 |
| NIHSS > 20 OR = 8.3 (2.4–32.6), p < 0.001 | ASPECT < 8 OR = 12.8 (2.0–81.6), p = 0.007 |
| Tandem lesion OR 6.1 (1.8–20.8), p = 0.004 | |
| Postdilatation balloon < 5 mm or absent* OR = 4.6 (1.2–17.6), p = 0.020 | |
| Peri-procedural DAPT initiation OR = 0.77 (0.41–0.92), p = 0.006 | |
| Balloon catheter use for cerebral protection OR = 0.68 (0.21–0.89), p = 0.003 |
Table VI
Predictors of stent patency loss by 90 days
| Univariate | Multivariate |
|---|---|
| Heparin limited to flush OR = 14.3 (1.5–53.1), p = 0.007 | Postdilatation balloon < 5 mm OR = 15.2 (5.7–72.3), p < 0.001 |
| mTICI < 2b OR = 12.7 (4.9–97.9), p = 0.001 | mTICI < 2b OR = 6.3 (0.98–45.2), p = 0.080 |
| Tandem lesion OR = 9.2 (1.1–28.4), p = 0.030 | |
| Postdilatation balloon < 5 mm* OR = 7.1 (5.4–57.9), p = 0.002 | |
| ASPECT < 8 OR = 6.2 (1.3–14.1), p = 0.024 |
* Includes lack of postdilatation (see Table II).
Discussion
The principal findings from this multicenter study in consecutive patients with acute stroke of carotid artery origin undergoing emergency revascularization treatment are that – despite variability in procedural strategies and pharmacotherapy – the MicroNET-covered anti-embolic stent use in an otherwise routine procedure is associated with (1) a high acute angiographic success rate, (2) high 90-day patency and (3) favorable clinical outcomes at 90 days.
So far, there has been only limited information on the performance of MicroNET-covered stents in carotid revascularization in acute ischemic stroke. The study by de Vries et al. [36] included only 5 non-consecutive patients while the pilot series by Klail et al. [37] and Tekieli et al. [20] analyzed outcomes in, respectively, 33 (non-consecutive) and 43 (consecutive) CRS patients with CGuard-EST. The present study is the first multi-center series of consecutive CRS patients treated with the MicroNET-covered stent.
The majority of CRS originate from carotid artery atherosclerosis [2, 7, 19, 22, 24, 51], with the platelet-rich lytic-resistant clot arising from the eroded or ruptured carotid plaque [52]. Part(s) of the clot may separate and cause “downstream” artery-to-artery embolism [51]. Less frequent CRS mechanisms involve (spontaneous or traumatic) dissection and (cardiogenic) embolism [2, 3, 7]. CRS lesions are either isolated extracranial (“non-tandem” stroke) or are “tandem” (extracranial carotid stenosis/occlusion plus intracranial artery occlusion). Epidemiologic data and clinical series of consecutive patients show that ~50% CRS are “non-tandem” while the other ~50% present as “tandem” occlusions [3, 14, 23].
Clinical manifestations of acute ICA occlusion are very variable [3]. This variability is related essentially to an interplay of three factors: the characteristics of occlusion (including its pace – gradual or abrupt), anatomic collateral circulation (and its functional susceptibility to exhaustion as a function of cerebral vasoreactivity) and hemodynamic factors [3]. Intracranial circulation involves a variable network connecting both anterior-posterior and deep-shallow circulation systems. Thus the compensation mechanisms in ICA occlusion are influenced by patient-specific anatomy and other preexisting factors. As a result, the clinical spectrum of ICA occlusion varies from a total absence of symptoms, through mild transient deficit, to a fully developed CRS in 40–50% of occlusions [3]. CRS, whether tandem or non-tandem, are often large [3] and are associated with poor clinical outcomes [3, 20]. CRS outcomes, whether tandem or non-tandem, are generally worse than those seen with isolated intracranial LVO [3–7, 20].
In some instances the extracranial lesion, rather than the primary stroke cause, is a bystander of cardiogenic thromboembolism [3, 53]; it adds significant hindrance to EST [3]. Fundamental challenges of CRS-EST include decision-making on at least ballooning the carotid stenosis (vs. leaving it unaddressed on an emergency basis) and/or stenting (vs. non-stenting) as well as the sequence and timing of the intracranial and extracranial intervention (antegrade vs. retrograde strategy). Operator decisions on procedure strategy and devices have important consequences for (administered or abandoned) pharmacologic therapy that, in turn, affect the cerebral and clinical outcomes [3, 16, 24]. Fundamental concerns of CRS EST are thus not only those shared with intracranial LVO EST, such as embolization to a new vascular territory and stroke extension (which are greater in CRS-EST than in intracranial LVO-EST) [7, 15, 25] and risk of symptomatic intracranial hemorrhage that is aggravated in CRS in relation to the cerebral infarct size and antiplatelet/antithrombotic therapies with stent use.
Aggregated data indicate that stenting of the carotid lesion (rather than balloon angioplasty only) improves overall outcomes in CRS [16, 19, 27]; thus stenting is today a preferred strategy. In a recent systematic review and meta-analysis comparing functional outcomes, reperfusion, sICH, and 90-day mortality of CAS versus balloon angioplasty alone in patients with tandem CRS, acute CAS and the retrograde approach had higher odds of successful reperfusion and good functional outcome [27]. Acute CAS was associated with higher odds of an mRS score ≤ 2 (odds ratio [OR] = 1.95, 95% CI: 1.24–3.05) and successful reperfusion (OR = 1.89, 95% CI: 1.26–2.83), with no differences in mortality or symptomatic intracranial hemorrhage rates [27]. Moreover, the retrograde (vs. antegrade) approach was significantly associated with a modified Rankin Scale score ≤ 2 (OR = 1.72, 95% CI: 1.05–2.83) [19]. A subsequent larger-scale meta-analysis, including 46 observational studies, confirmed a higher good functional outcome of acute CAS compared with the no-stenting approach (OR = 1.52, 95% CI: 1.19–1.95), and higher successful recanalization rate (OR = 1.91, 95% CI: 1.29–2.85), with a lower restenosis rate in the acute CAS group than in the no-stenting group (2% vs. 9%, p = 0.001). Overall, the recanalization rate was higher in retrograde than antegrade CAS (OR = 0.51, 95% CI: 0.28–0.93) [16]. In the third recent meta-analysis, after adjustment for confounders, the odds of a favorable functional outcome (adjusted odds ratio [aOR] = 1.67; 95% CI: 1.20–2.40; p = 0.007), favorable shift in mRS scores (aOR = 1.46; 95% CI: 1.02–2.10; p = 0.04), and successful reperfusion (aOR = 1.70; 95% CI: 1.02–3.60; p = 0.002) were significantly higher for the CAS group compared with the non-stenting group. Both groups had similar odds of sICH (aOR = 0.90; 95% CI: 0.46–2.40; p = 0.87) [19].
Although stenting (vs. no stenting) is associated with improved outcomes of CRS-EST, with conventional carotid stents there is a clinically significant risk of stent thrombosis and occlusion. The 20–30% risk of new embolism with single-layer stents translates into their poor (or absent) postdilatation optimization [7, 25, 28, 29], while poor stent embedding, residual stenosis and too small stent diameter are well-known factors of patency loss [30]. Intravascular imaging confirms that conventional carotid stents may fail to insulate the athero-thrombotic material in elective CAS [54, 55]; thus they are challenging to optimize. This limitation is even more relevant with the large thrombo-embolic load typical for CRS. Stent underexpansion and malapposition, along with the challenges of antiplatelet and antithrombotic management in CRS, result in increased rates (in relation to elective stenting) of symptomatic thrombosis and re-occlusion. Registry data show that with 1st generation stent use in CRS the risk of stent occlusion may exceed 30% [25]. Data from the Endovascular Treatment In Stroke (ETIS) Registry of CRS-EST in tandem strokes show that stent post-dilatation (even if not optimized by our criteria) increases the likelihood of stent patency at 24 h by 2.42-fold (95% CI: 1.24–4.67) [28]. Although the success of intracranial LVO revascularization (final TICI flow) plays a fundamental role, optimal stent embedding may be highly relevant because post-procedural loss of stent patency in CRS-EST is associated with a significant negative impact on the likelihood of a good functional outcome at 3 months [56].
There is increasing understanding of the role of cerebral protection when performing EST in LVO strokes; this appears to be particularly relevant to CRS due to its generally large thrombus burden [57]. The STRATIS registry of over 500 patients indicated that the use of a balloon guide catheter versus a conventional catheter was an independent predictor of first-pass effect, modified first-pass effect (mTICI ≥ 2b) and functional independence [58]. The recent propensity score-matched analysis of a series of tandem CRS by the Buffalo Group [57] demonstrated that the patients with a balloon guide catheter had a significantly shorter procedure duration, lower discharge NIHSS score, and higher odds of 90-day mRS 0-2. On multivariate regression, the balloon guide catheter cohort had a significantly higher first pass effect rate and lower periprocedural sICH rate. Dynamic flow reversal in transcarotid artery revascularization (TCAR) is very effective in removing atherothrombotic debris from the treated lesion. There is evidence for an additive neuroprotective effect of the TCAR robust flow reversal and the MicroNET-covered anti-embolic stent that limits intra-procedural embolism and prevents post-procedural plaque-related embolism [59]. The present study indicates that TCAR can be effectively used in patients with acute CRS, in particular in cases of failure of transfemoral/transradial access (Table II, Figure 3). To be able to use the transcarotid access on an emergency basis the team needs to be proficient in gaining this access safely and swiftly; IVT received by the patient is an important limiting factor. Recent data in elective procedures of carotid revascularization suggest an additive benefit of TCAR dynamic flow reversal (allowing a safe procedure of stent implantation and optimization) and the anti-embolic stent whose protection against the atherothrombotic plaque-related embolism extends to the post-procedural period. In the present study a cerebral protection device was applied in 45.3% of procedures, with proximal system use in 38.7% of study participants (Table II), potentially contributing to favorable clinical outcomes. On univariate analysis, balloon guide cerebral protection significantly reduced the likelihood of bad clinical outcome at 90 days (OR = 0.68, 95% CI: 0.21–0.89, p < 0.005); however, it was not an independent predictor on multivariate analysis (Table V).
While stent use (according to instructions for use and clinical experience) requires both anticoagulation and antiplatelets, any antithrombotic regimen is believed to increase the likelihood of stroke hemorrhagic transformation and is intensively debated [60]. Heparin and antiplatelet use in CRS-EST are not standardized. For heparin, the doses range from zero (i.e., not even in catheter flush) to a “full” (ACT-adjusted) dose [21, 46, 48]. Recent analysis from the TITAN Collaboration indicated that periprocedural unfractionated heparin did not modify the safety and efficacy results of emergency CAS in tandem strokes [61] but Da Ros et al. [48] suggested that heparin dosage ≥ 3000 IU increased the sICH rate in CRS-EST when the initial ASPECTS was ≤ 7 and intracranial mechanical thrombectomy required more than one passage for complete recanalization. In our study, the minimal heparin use was that limited to catheter flush; otherwise it reflected the practice variability (Table II). Heparin other than in flush was a predictor of sICH on univariate analysis but it did not constitute an independent predictor (Table IV).
Absence of double antiplatelet therapy (DAPT) and of ACT-adjusted heparinization may be particularly relevant for stent thrombosis in thrombus-containing lesions and with post-recombinant tissue plasminogen activator (rtPA) rebound thrombogenicity in CRS. In addition, stent undersizing and underdeployment (major stent thrombosis contributors [30]) are more likely to occur in a highly thrombotic milieu because of the difficulty in true vessel diameter determination (note increased likelihood of the internal carotid artery under-filling/constriction) and the concern of a potential increase of the thrombus intraluminal migration with stent diameter optimization by up-sized balloon postdilatation(s) [62]. In the extremely challenging CRS-EST setting, a happy medium may not exist between providing the appropriate CAS-required heparinization level and early DAPT administration while, in turn, enhancing the risk of stroke hemorrhagic transformation [62].
According to a recent meta-analysis by Diana et al. [16] stenting (vs no-stenting) in tandem CRS significantly increased risk of sICH (OR = 1.97, 95% CI: 1.23–3.15). However, intraprocedural antiplatelet use during emergency CAS was associated with a higher rate of good functional outcome (60% vs. 46%, p = 0.016) and lower rate of sICH (7% vs. 11%; p = 0.08) compared with GP IIb/IIIa inhibitors, indicating that GP IIb/IIIa inhibitors might play a significant role in the increase in sICH. This is consistent with our data (Table IV). With conventional carotid stents, high-intensity antiplatelet regimens (DAPT or GP IIb/IIIa inhibitors) are likely to positively affect result in stent patency compared with low-intensity regimens [16, 45, 48]. Preliminary data suggest that low-dose intravenous cangrelor has similar safety and a higher rate of complete reperfusion compared to IV GP-IIb/IIIa inhibitors while it may be safer (elimination half-life 3–6 min) [47]. Recent evidence indicated that – in the case of CGuard use with an optimized implant embedding – delayed administration of the second antiplatelet may not translate into any increase in the risk of stent thrombosis [63]. The CGuard self-tapering feature (‘SmartFit’) [31, 64] (Figures 1, 2, 5) may, by the device natural fit to the anatomy and thus a reduced risk of malapposition (particularly when post-dilatation is performed), play an important role in minimizing the risk of stent thrombosis.
Evidence shows that MicroNET-covered stent, an important development in the evolution of carotid revascularization devices and techniques [32, 64–66], contributes to increasing the safety and quality of elective CAS [67]. Findings from the present study suggest that the device combining the most “open-cell” (nitinol frame) design with the ultra-thin (20 μm) most “closed cell” design (MicroNET wrap with pore size of ≈150–180 μm, i.e., the range of the pore size in filters used for cerebral protection in CAS) may be similarly likely to contribute to an increased penetration of quality emergency endovascular recanalization in carotid-related stroke, significantly reducing the burden of disability, dependence and mortality [3]. Optimized embedding of the neuroprotective MicroNET-covered stents into the vessel wall reduces the risk of implant thrombosis [30, 31, 33, 62].
A recent large-scale meta-analysis of clinical data with new para (single-layer) and “mesh” stents indicated that marked differences in the mechanical properties of the distinct “mesh” stent types may translate into clinically relevant differences in 30-day and 12-month outcomes [67]. In elective CAS, CGuard clinical outcomes are superior to 1st-generation (single-layer) carotid stents at 30 days (reduction in death and stroke) and 12 months (reduction in both ischemic stroke and in-stent restenosis) [67].
Clinical and angiographic outcomes in this multi-center study of CGuard stent use in CRS-EST appear superior to those recently published by Klail et al. [37], who reported results in 33 non-consecutive patients with tandem and non-tandem CRS. In that study, the mortality rate was 24% with a favorable clinical outcome (mRS ≤ 2) in 36% of patients at 90 days [37]. However, ASPECTS on admission (median: 7) was lower than that in our patients (median: 9). Also, the MicroNET-covered stent size matrix of devices used in that study includes stent size diameter of 4 mm [37] while the smallest CGuard stent diameter is 6 mm, indicating the potential use of stent types other than CGuard within the series reporting CGuard outcomes [37]. Reduction in the risk of unfavorable outcome at 90-days with the use of balloon guide catheters for cerebral protection in CRS-EST (Table V) is consistent with the benefit of proximal cerebral protection in elective CAS [68]. Because the anti-embolic stent exerts its neuroprotective action only from the point of the device full implantation (but with neuroprotection against lesion-related extending into the stent healing period [34]) the benefit of cerebral protection prior to stent placement and optimization is additive to the anti-embolic stent cerebral benefit.
Limitations: Our protocol recommended, on the basis of CGuard evidence in elective CAS [31, 33], post-dilatation device embedding in the artery wall, but other elements of the procedural strategy, such as the sequence of stenting (antegrade/retrograde) and antiplatelet regimens, were not standardized. Yet, with routine post-dilatation optimization the study device performed well (high 90-day patency rate, high rates of good and excellent clinical outcome) despite variability in procedural strategies and pharmacotherapy. As < 50% of stroke patients eligible for mechanical reperfusion may be eligible for inclusion in clinical studies with strict protocols [49], our all-comer eligible patient enrollment and the operators’ freedom to use (apart from the study device implantation technique that recommended post-dilatation) their routine devices and strategies translate into an important advantage of our study in relation to its relevance to routine clinical practice. Nevertheless, larger multi-center series and longer follow-up data [69] are warranted.
Conclusions
This MicroNET-covered stent study in consecutive CRS patients eligible for mechanical reperfusion – the largest such study to date – demonstrated a high acute angiographic success rate, high 90-day patency and favorable clinical outcomes despite differences in procedural strategies and pharmacotherapy, reflecting real-life variability in approaches by different operators and centers. The MicroNET-covered stent was beneficial in ICA recanalization and re-establishing cerebral perfusion with minimized iatrogenic cerebral embolism, and was associated with a high rate of functional independence. Study stent post-dilatation with a small-diameter balloon (or absence of post-dilatation) independently predicted patency loss; this – taken together with the minimal in-stent restenosis rate with the device post-dilatation optimization strategy in elective CAS [31] – calls for a strategy of MicroNET-covered routine post-dilatation optimization in emergency carotid revascularization. In anticipation of larger-scale data, findings from this study may inform selection of the management strategy in patients with acute ischemic stroke of carotid artery origin.








