Summary
We conducted this study to predict the need for permanent pacemaker implantation after transaortic valve implantation. In our study, a relationship was found between aortic knob calcification, which can be easily seen through chest radiography, and the patients’ need for permanent pacing after the procedure. This demonstrated the usefulness of a parameter that provides important results in terms of both increasing the precautions that operators can take before the procedure and informing patients about complications after the procedure.
Introduction
Transcatheter aortic valve implantation (TAVI) is a treatment option that was initiated with animal experiments 30 years ago in the treatment of advanced aortic stenosis and has been used safely in humans for 20 years [1, 2]. Although TAVI is generally recommended for patients with moderate and high risk for surgery, data on interventions for patients with low surgical risk are available in the literature [3–5]. Although it is considered to have a lower risk than surgical aortic valve replacement, TAVI is associated with various complications, including peripheral vascular complications, conduction abnormalities, myocardial infarction, stroke, and death [6–8]. In the PARTNER study, the rate of conduction abnormalities after TAVI was determined to be 34.8% [9]. Among the reported abnormalities are left bundle branch block (LBBB), right bundle branch block (RBBB), hemiblock, intraventricular conduction delay, first-degree atrioventricular (AV) block, and high-degree block on electrocardiography [10]. LBBB is the most common conduction disorder due to anatomical proximity [11].
It is recommended to implant a permanent pacemaker (PPM) in the presence of a high-degree AV block or a newly developing alternant bundle branch block 24–48 h after the TAVI procedure [12]. If the conduction problem worsens in patients with RBBB before the procedure or for those with newly developed LBBB (QRS > 150 ms or PR > 240 ms) and there is no further prolongation for > 48 h after the TAVI, then the use of a pacemaker should be considered [12]. Many electrocardiographic, demographic, anatomical, and procedural parameters have been found to predict the requirement for a PPM after TAVI [13–16]. One of the anatomical parameters is the presence of a porcelain aorta [17].
There is a strong relationship between aortic calcification and aortic valve annular calcification [18]. Aortic valve annular stiffness has been shown to be one of the main causes of damage to the conduction pathways by increasing the level of mechanical trauma during the TAVI procedure [19].
Aim
In this study, we investigated the relationship between aortic knob calcification (AKC), assessed by preoperative chest X-ray as a cost-effective and easily accessible method, and the requirement for PPM implantation after TAVI.
Material and methods
Study design and population
This retrospective study enrolled patients who underwent TAVI at a center experienced in TAVI between June 2020 and December 2022. Data on 180 patients were obtained. The distribution of the patients according to the heart valve used was as follows: Myval, n = 116; ACURATE neo, n = 41; Medtronic Evolut R, n = 18; Edwards SAPIEN 3, n = 3; and St. Jude Medical Portico, n = 2. In order to eliminate procedural variations, considering the number of patients in each group, only those who received a Myval valve (n = 116) were initially included in the study group. Upon further examination, 6 of these patients were excluded from the study due to four having permanent pacemakers and two having a high-degree AV block before the procedure. The demographic data of the remaining 110 patients were recorded for pre- and post-procedure electrocardiograms (ECGs) and PPM processes, together with echocardiography reports, laboratory parameters, pre-procedural chest radiographs, and hospital follow-up notes. The patients’ complete blood count, kidney function tests, fasting blood glucose levels, and lipid panel were also recorded. Pre-and post-procedure ECGs were assessed by two independent cardiologists, with the results being interpreted according to the current guidelines in terms of each patient’s requirement for a PPM after TAVI [12].
Posterior-anterior chest radiography
The chest radiographs of the patients were examined and graded for AKC, as described in recent studies [20]. The examination was performed using the following grading system: Grade 0, no calcification; Grade 1, point calcifications or small fine lines of calcification; Grade 2, thickened calcification in one or more areas; and Grade 3, circular thickened calcification (Figure 1).
Echocardiography
The echocardiographic examination was performed using the Philips IE33 system (Philips Medical Systems, Andover, MA, USA). The left ventricular ejection fraction was calculated using Simpson’s method. The interventricular septum, left ventricular end-diastolic diameter, end-diastolic diameter, and left ventricular outflow tract (LVOT) diameter were calculated using M-mode echocardiography from the parasternal long axis window. Aortic velocity, aortic valve peak, and mean gradient were calculated using continuous wave Doppler from the apical 5-chamber window. The aortic valve area was calculated using the continuity formula. Mitral regurgitation was graded from the apical 4-chamber window, and the systolic pulmonary artery pressures over the tricuspid valve were calculated.
Computed tomography (CT)
Images were recorded using a multi-detector CT device (Siemens Somatom Sensation 64 Cardiac) following the injection of 120 ml of iohexol in patients with suitable kidney functions. The images recorded during the evaluations conducted by specialized and experienced cardiology and radiology physicians together were analyzed. Three-dimensional images were evaluated, and aortic valve structure, annulus measurements, coronary arteries, the sinus of Valsalva, and the LVOT were evaluated. The length of the membranous septum (MS) was calculated using coronal images. A threshold of 850 Hounsfield units was used to determine the calcium score in the valve on contrast-enhanced scans. Coronary angiography was planned for patients with moderate and severe stenosis on coronary CT. Revascularization was performed on patients with severe stenosis on coronary angiography, and the aortic stenosis treatment protocol was resumed 6 months later according to their symptoms and complaints.
TAVI procedure
The transfemoral pathway was preferred for implantation. All procedures were carried out by the same operator team and experienced healthcare personnel specializing in TAVI. The procedures were completed by heparinizing under conscious sedation, ensuring that the active coagulation time was > 250 s. During the implantation procedure, the valves were inserted using angiography in the annular plane, with 70% placed in the aortic area and 30% in the ventricular area, following the Myval valve landing zone. In cases where RBBB was detected in the baseline ECG, efforts were made to prevent deep placement. Peripheral hemostasis was achieved using Prostar XL or Perclose ProGlide 6-Fr suturing devices.
Statistical analysis
The statistical analysis was performed using SPSS Statistics v. 20.0 for Windows (IBM Corp.). The patients were divided into two groups: those who required a post-procedure PPM and those without this requirement. In order to examine the parametric and non-parametric distributions of the data, the Kolmogorov-Smirnov and homogeneity of variance tests were performed. The independent-samples t-test was used to compare two groups of variables showing a parametric distribution, while the Mann-Whitney U test was employed to compare two groups of variables without a parametric distribution. The categorical variables were compared using the χ2 test. Parametric continuous variables were expressed as the mean ± standard deviation (SD). Non-parametric variables were expressed as median (interquartile range [Q1–Q3]) values. Categorical variables were presented as numbers and percentages. Univariable and multivariable logistic regression analyses were performed to determine the predictive parameters of PPM implantation after TAVI. Two methods were used for multivariable regression analysis. First, the parameters in the entire dataset were entered into univariable regression analysis to determine whether they were dependent predictors of the requirement for PPM after TAVI. In univariable regression analysis, parameters with a p-value of < 0.05 were considered statistically significant, while those with a p-value of > 0.05 were not deemed to be statistically significant. Then, the parameters that were identified as dependent predictors by the step-by-step regression analysis were included in the multivariable regression analysis (Model A). Second, regardless of the univariable regression analysis, we included all the parameters in our dataset that were previously associated with the requirement for PPM after TAVI in the literature, as well as our new parameter of interest in multivariable regression analysis (Model B). The correlation between the parameters determined as independent predictors in the multivariable regression analysis and the requirement of PPM after TAVI was investigated using the Pearson and Spearman correlation tests.
The study protocol was approved by the local ethics committee in accordance with the principles of the Declaration of Helsinki and good clinical practice.
Results
The study included a total of 110 patients with aortic stenosis who underwent TAVI with a Myval valve (Meril Life Sciences, Gujarat, India). The mean age of the patients was 79.92 ±7.57 years. The rate of pacemaker implantation after TAVI was found to be 15.4%. Following TAVI, 17 patients (8 women and 9 men; mean age: 80.64 ±6.05 years) were found to require PPM. The remaining 93 patients who did not require PPM (42 females and 51 males; mean age: 79.78 ±7.84 years) were evaluated as the control group. Of the 17 patients in the PPM group, 12 were fitted with a dual-chamber (DDD) pacemaker, four with a single-chamber (VVI) pacemaker, and one was fitted with a cardiac resynchronization therapy defibrillator (DDD mode). When the PPM requirements of other valve models excluded from the study were examined, it was found that this rate was 12% (n = 5/41 patients) for those who received ACURATE neo and 5.5% (1/18 patients) for those who received Medtronic Evolut R. There were no PPM requirements among any of the cases in which the Edwards SAPIEN (n = 3) or the St. Jude Medical Portico (n = 3) was used.
The demographic and laboratory data of the patients included in the study, as presented in Table I, were compared, and no significant differences were found between the PPM and control groups. Table II shows the comparison of the echocardiography, electrocardiography, chest X-ray, and CT data between the groups. The distribution of AKC among the patients in both groups was also examined (Figure 2).
Table I
Clinical characteristics and laboratory parameters of the study groups
[i] TAVI – transaortic valve implantation, SD – standard deviation, F – female, M – male, BMI – body mass index, CAD – coronary artery disease, CKD – chronic kidney disease, COPD – chronic obstructive pulmonary disease, CVD – cerebrovascular disease, HDL-C – high-density lipoprotein cholesterol, Hs-troponin I – high-sensitivity cardiac troponin I, LDL-C – low-density lipoprotein cholesterol, PPM – permanent pacemaker, WBC – white blood cells.
Table II
Comparison of the echocardiography, electrocardiography, and computed tomography parameters
[i] TAVI – transaortic valve implantation, AVA – aortic valve area, IVS – interventricular septum, LVEF – left ventricular ejection fraction, LVEDD – left ventricular end-diastolic diameter, LVESD – left ventricular end-systolic diameter, LVOT – left ventricular outflow tract, SD – standard deviation, RCC – right coronary artery, LMCA – left main coronary artery, NCC – non-coronary cusp, PAPs – pulmonary arterial pressures systolic, PPM – permanent pacemaker, RBBB – right bundle branch block, STS – Society of Thoracic Surgeons.
Figure 2
Distribution of aortic knob calcification in groups with and without a permanent pacemaker (PPM) requirement after transaortic valve implantation
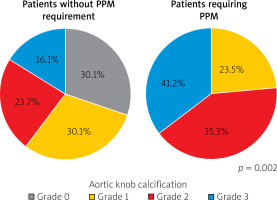
The rates of MS (p = 0.013) and AKC (p = 0.002) statistically significantly differed between the PPM and control groups. Table III presents the comparison of the procedural characteristics of the patients, the values obtained after the TAVI procedure, and the mortality results evaluated over an average period of 18 ±4 months. There were no significant differences between the two groups in terms of these parameters.
Table III
Comparison of procedural and post-procedural parameters and evaluation of short-to-mid-term mortality outcomes
Univariable regression analysis performed to identify predictors of PPM implantation after TAVI is shown in the left column of Table IV. According to the results, MS (p = 0.013) and AKC (p = 0.004) were dependent predictors of the PPM requirement after this procedure. In the middle column (multivariable analysis Model A), both parameters that were dependent predictors in univariable regression analysis were also determined as independent predictors (p = 0.039 for MS, p = 0.024 for AKC). The left column of the table presents multivariable analysis Model B, in which the parameters associated with the PPM requirement after TAVI in the literature were included, and independent predictors were determined to be MS (p = 0.027) and AKC (p = 0.040) among a wider range of factors. Correlation analyses were performed to determine the relationship of the post-TAVI PPM requirement with AKC (correlation coefficient: 0.595, p = 0.002) and MS (correlation coefficient: –0.496, p = 0.01).
Table IV
Univariable and multivariable analyses showing the relationship between parameters and PPM requirement after TAVI
Discussion
In this study, we examined the relationship between the requirement for PPM after Myval transcatheter heart valve (THV) (Meril Life Sciences, Gujarat, India) implantation as a balloon expandable TAVI system and the presence of AKC identified on chest X-rays during the preoperative evaluation. We found that, in our cohort, AKC and MS were both dependent and independent predictors of the requirement for PPM after TAVI.
In order to ensure a more homogeneous patient group, we specifically focused on the Myval THV valves in our study. Supporting our hypothesis, a review published by Szotek et al. in 2019 indicated that the differences in PPM implantation rates after TAVI were mostly related to the valve system used [21]. Our aim was to determine the relationship between AKC and the PPM requirement while excluding the variations caused by different valve systems.
The three main causes of conduction abnormalities after TAVI are mechanical trauma, medical treatments, and unchangeable anatomical and electrical changes in the patient [19]. Mechanical trauma is minimized by ensuring that the operators are attentive and considering patient-oriented factors, such as the procedure and valve selection. Thus, extensive research and studies have focused on these parameters. Factors such as LVOT stiffness, deeper implantation, and the use of a larger balloon or valve have been investigated. LVOT stiffness has been shown to be an important parameter in assessing mechanical trauma. The mechanism of mechanical trauma can be explained by the effect of the aortic annulus, surrounding structures, and the prosthesis being implanted. This process involves patient-dependent factors, such as LVOT calcification [22] and valve calcification [23], as well as procedural factors, including pre- and post-dilatation [24] and the preference of a large balloon or valve [25].
Damage to the conduction pathways and the requirement for PPM after TAVI are inevitable due to the anatomical proximity of the aortic valve, AV node, and bundle of His. The close contact between the non-coronary cusp (NCC) and conduction pathways is particularly noteworthy, as it is the primary factor leading to a higher incidence of AV block in TAVI cases with NCC calcification [22, 26].
In our study, no relationship was found between the PPM requirement and aortic valve calcification, LVOT calcification, or NCC calcification evaluated on CT. We consider that the high rate of NCC calcification in all patients may have prevented a statistically significant difference. This evaluation may provide more accurate results when performed on datasets where there is a higher number of patients and NCC calcification is distributed in a more balanced way.
The MS is in contact with the right coronary cusp and NCC regions of the aortic valve. The bundle of His divides into right and left bundles by piercing the septum from the distal muscular septum origin of the MS. A shorter MS length increases the contact between the prosthetic valve and the bundle of His, leading to more conduction damage [27]. In our study, a strong relationship was found between PPM and MS length after TAVI (6.8 [3.1–14], 10.35 [6.03–15.7]; p = 0.013).
Another cause of mechanical trauma is the implantation site. In our study, although the implantation area was determined to be 70–30% according to the Myval THV landing zone used as standard, we opted for higher placement in selected cases, especially in the case of RBBB in the baseline ECG. Therefore, the presence of RBBB in the baseline ECG [28], which is considered a strong indicator for the PPM requirement after TAVI, did not show statistical significance in our study.
Demographic characteristics have an effect on the PPM requirement after TAVI. Age generally appears to be a significant factor. However, our study indicated that age was not a significant factor in distinguishing between the PPM and control groups. According to a relevant study undertaken by Ravaux et al., analyzing the data from 7,489 patients, the mean age was 80 years for the PPM group and was 79.7 years for the control group [29]. Although this shows the numerical consistency between these data and our data, our small sample size prevented the observation of any statistical difference. Similarly, the authors of the previous work reported the rate of male patients to be 54.9% in the PPM group, which aligns with the rate found in our study (53%).
Current studies have proven the importance of AKC, evaluated using a chest X-ray, as a reliable predictor and a prognostic marker for cardiovascular diseases [21, 30–32]. In addition, in a study by Korkmaz et al., the relationship between AKC and systemic arterial stiffness was demonstrated [33]. In another study, Allison et al. reported a correlation between systemic arterial stiffness and aortic annulus calcification [18]. Patients with aortic stenosis experience a significantly higher rate of calcification around the aortic valve. In our study, grade 3 (median) aortic calcification was detected in both the PPM and control groups. This finding indicates reduced sensitivity in the prediction of which patients undergoing TAVI may require PPM. However, our purpose in this study was to focus on the aortic arch region rather than the aortic valve region since this allows observation of the dissemination and systemic nature of calcification, reaching a level of stiffness that could increase the effect of mechanical trauma. Consequently, we were able to present a parameter that can be evaluated with a simple chest X-ray. AKC is of clinical importance as a prominent indicator for operators to adjust modifiable factors related to mechanical trauma.
AKC and porcelain aortas attract attention with their intertwined definitions. Porcelain aorta is a structural aortic wall disease characterized by widespread, severe calcification of the thoracic aorta. It can be identified during surgery, after a sternotomy, or by use of CT before the procedure. However, it is much more appropriate to define AKC rather than the porcelain aorta using a chest X-ray. Although these two terms share similarities, they are not synonymous. The primary objective of our study was to demonstrate that AKC grading can be established accurately via chest X-ray, which is one of the simplest examinations available.
Our study revealed that AKC was both a dependent predictor in univariable regression analysis and an independent predictor in two distinct multivariable regression analysis models. Model A included two parameters that were statistically significant according to the univariable regression analysis, while a more comprehensive model was constructed with Model B by incorporating all the parameters identified as PPM predictors after TAVI in the 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy [12].
In our study, the PPM requirement rate after TAVI was found to be 15.4%. In a previous study, it was found that the PPM requirement was lower with the use of balloon-expandable valves compared to self-expandable valves [34]. Considering balloon-expandable valves, in the PARTNER 2 study, the rate of PPM implantation following the use of SAPIEN 3 was reported to be 13.3% [35]. In a study evaluating the outcomes of the use of the SAPIEN XT valve, the rate of the PPM requirement was found to be 9.5% for this valve [36]. When evaluating data related to the Myval valve, a recent study by Delgado-Arana et al. found the rate of PPM requirement to be 15.5% for the SAPIEN 3 and 5.8% for the Myval valve [37]. In studies examining the outcomes related to the use of the Myval valve alone, Santos-Martinez et al. determined the PPM rate to be 7.4% in 135 patients, while Akyüz et al. found it to be 8% [38]. In another study conducted by Barki et al., the PPM rate was found to be 11% [39]. In an international study published in March 2024, Kilic et al. reported that 12.1% of patients required PPM over a 2-year follow-up after Myval valve implantation [40]. The lowest PPM rate reported to date in the literature is 5.8%. In our study, the observed PPM requirement after TAVI was 15.4%.
We detected no significant correlation between procedure time, valve size, and PPM requirement. In previous studies, a correlation was established between the rates of prosthetic valve implantation and aortic annulus and PPM requirement. However, our study did not identify any correlation between PPM and the calculation obtained by dividing the valve prosthesis area by the aortic annulus area. This may be attributed to nine different sizes being available in the Myval THV system (20 mm, 21.5 mm, 23 mm, 24.5 mm, 26 mm, 27.5 mm, 29 mm, 30.5 mm, and 32 mm), and the appropriate valve size is chosen according to the annulus measurements on the CT scan of each patient before the procedure. In our study, the ratio of the valve area to the aortic annulus area had mean values of 1.06 and 1.05 for the PPM and control groups, respectively, supporting our hypothesis. Among the 17 patients in the PPM group, a DDD pacemaker was implanted in 13, and a VVI pacemaker in four. While 9 patients presented with atrial fibrillation in the PPM group, it was clinically decided to implant a DDD pacemaker for sinus rhythm control in five patients.
There is currently no clear consensus on the effect of PPM implantation on mortality after TAVI among the studies in the literature. While Xu et al. reported an increase in all-cause mortality in a meta-analysis, they did not detect any changes in mortality attributed to cardiovascular causes [41]. In contrast, in a study by Engborg et al., the mortality rate was found to be lower among patients who underwent PPM implantation after TAVI [42]. In our study, no significant difference was found between the groups with and without the PPM requirement in relation to the in-hospital mortality rate, the mean 18-month cardiovascular mortality, or total mortality.
Study limitations. This study had a single-center design and was conducted with a limited number of patients. Multi-centered studies and meta-analyses can yield more insightful results on this matter. In addition, our study focused on a single-model valve system, which is the most frequently used in our clinic in daily practice. Therefore, the findings may not be generalizable to other valve systems, representing a limitation of our study.
Conclusions
In this study, we determined that AKC, which can be detected in a simple and cost-effective manner through a chest X-ray, was able to predict the requirement for PPM after TAVI. Given the accessibility of this imaging modality for every patient, the identification of AKC may reduce PPM rates by positively affecting the procedural methods of the operator and valve selection.