Introduction
Angiomyomatous hamartoma of the lymph node (AMH-LN) is an extremely rare, benign vascular disease of unknown etiology [1], which is characterized by partial or complete replacement of the lymph node parenchyma by irregularly distributed thick-walled blood vessels, smooth muscle bundles and adipose tissue in a fibrotic stroma [2]. It was described for the first time by Chan et al. in 1992 [3], and to our knowledge, there are 70 cases described in the literature to date. Angiomyomatous hamartoma (AMH) occurs mainly in inguinal and femoral nodal regions, but there are a few reports of some other locations – submandibular, cervical, popliteal and paraaortic lymph nodes [4–8]. We present a case of a 37-year-old female patient with AMH-LN in the pelvic and paraaortic lymph nodes who presented with weight loss.
Case report
A 37-year-old woman was referred to our clinic due to weight loss – 7 kg in 7 months, moderate pelvic pain syndrome and suspected pelvic tumor. Upon admission to the Gynaecologic Oncology Department, her weight was 39 kg, and BMI was 16.23. The patient’s medical history record showed bronchial asthma and two previous Cesarean section – in 2007 and 2014. The laboratory tests and serum tumor marker (Ca-125) were normal. There was no evidence of acute or chronic inflammatory disease. The gynecological examination of the pelvis revealed a soft, poorly mobile tumor with unclear borders measuring 5/5 cm. It was assumed to be an ovarian tumor on the left. Vaginal ultrasound was performed: a normal uterus and adnexa were visualized; an echo-homogeneous tumor on the right, with smooth external and internal walls measuring 40/45 mm, was found. Computed tomography (CT) scan of the pelvis and abdomen was performed.
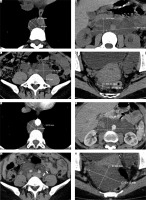
Computed tomography revealed numerous pathologically enlarged bulky lymph nodes in the paracaval and paraaortic area in size up to 44/109 mm (Fig. 1 A, B). Bulky lymph nodes on the right, paralytic, with sizes up to 37/24 mm, were detected. Bulky lymph nodes parallel to the left with 38.4/44.4 mm dimensions were visualized (Fig. 1 C, D).
Fig. 1
Computed tomography images before and 6 months after the surgical procedure. A) Paraaortic lymph nodes in the thoracic segment before surgery; B) bilaterally paraaortic lymph nodes in clusters at the level of the renal vessels before surgery; C) left parailiac lymph nodes before surgery; D) right parailiac lymph nodes before surgery; E) paraaortic lymph nodes in the thoracic segment after surgery; F) bilaterally paraaortic lymph nodes in clusters at the level of the renal vessels after surgery; G) left parailiac lymph nodes after surgery; H) right parailiac lymph nodes after surgery
A decision for surgical treatment was made in order to remove the described tumor and biopsy of the enlarged lymph nodes.
After standard preoperative preparation, a lower middle laparotomy was performed. Normal uterus and adnexa were visualized. Retroperitoneally on the left, a soft suspected enlarged lymph node with unclear boundaries was palpating, reaching the level of the bifurcation of the left common iliac artery with a size of 10/10 cm, which could not be differentiated by the pelvic vessels. This suspected enlarged lymph node was removed, leaking chylous contents. A lymphoproliferative process was intraoperatively diagnosed, which necessitated the completion of the intervention.
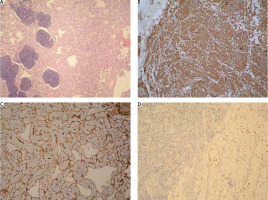
The material was represented by a poorly defined mass with partially preserved lymphoid tissue, replaced mainly by muscle-type vascular structures with pronounced smooth muscle hyperplasia in the wall, atrophic lymph follicles and mature adipose tissue mixed in different proportions, but with a predominance of the vascular component (Fig. 2 A). The latter is represented by channels covered by a single endothelium and filled with erythrocytes and fibrin. In immunohistochemical examination, the smooth muscle cells showed a diffuse positive reaction for smooth muscle actin (Fig. 2 B), positive expression for CD31 was observed in endothelial cells (Fig. 2 C), and the adipose tissue showed a positive reaction for S100 (Fig. 2 D). A negative reaction was reported for HMB-45, melan A, and a slightly positive reaction for estrogen. No necrosis, cytological atypia or mitosis was detected. Outside the compact vascular component, the blood vessels were deformed with hypertrophied and hyalinized media.
Fig. 2
Histological findings. A) Angiomyomatous hamartoma (AMH) – haphazardly arranged, fully formed, thick-walled, muscular blood vessels, surrounded by smooth muscle cells and fibrous stroma, occasional focal normal adipose tissue, and atrophic lymphoid tissue, HE 100×; B) AMH – smooth muscle actin immunostaining reveals the presence of numerous smooth muscles in the blood vessel wall and intervening spaces, immunohistochemistry – IHC, 100×; C) AMH – CD31 immunostaining stains the vascular endothelial cells and highlights the rich vascularity of the lesion, IHC 100×; D) AMH – S100 immunostaining expression fat component, IHC 100×
A benign lesion closest to AMH of the iliac lymph nodes on the left, a variant of AMH, was established based on morphology and immunohistochemistry examination.
The patient was provided with an abdominal drain for 48 hours and was discharged on the 5th postoperative day in good health condition.
At a follow-up examination 3 months later, the patient had regained 6 kg of her weight with a BMI of 18.73 and reported no pain syndrome. Six months after the operation, follow-up CT of the pelvis and abdomen was performed. The CT revealed persistence of the described paraaortic lymph nodes with a slight increase in size (Fig. 1 E, F), absence of tumor in the area of the left pelvic lymph nodes (Fig. 1 G) and persistence without resizing the right pelvic lymph nodes on the right (Fig. 1 H).
Discussion
Angiomyomatous hamartoma of the lymph node is a rare, benign disease that, according to the literature, affects men more often than women in a ratio of 2 : 1 [9]. Our search does not confirm the data. In the literature, we found 38 cases of men and 31 of women, including the present case. Angiomyomatous hamartoma of the lymph node most often involves the inguinal or femoral lymph nodes, rarely affecting more than one. Other locations have been described as well – 4 cases of lymph node involvement in the pelvis, only 1 in women [6–10], 3 cases of cervical lymph node involvement [4, 11, 12], 2 cases of post-auricular lymph node involvement, both in men [13, 14] and 3 cases in men with paraaortic and/or pelvic lymph node involvement [2, 15, 16]. The pathogenesis of this disease is unclear, suggesting that it may be a secondary response to inflammation of the lymph nodes responding to vascular proliferation [9].
The disease is usually asymptomatic only with swelling of the relevant lymph node, except for the popliteal or post-auricular lymph nodes. In these cases, a pain syndrome can be observed [8, 13]. In a case involving the paraaortic lymph nodes, nausea and reflux were observed [2]. Angiomyomatous hamartoma of the lymph node may cause swelling of the affected limb [3, 20, 22, 30, 31].
Table 1 presents the clinical characteristics of all women with this disease described in the literature.
Table 1
Clinical characteristics of women with angiomyomatous hamartoma of the lymph node described in the literature
| Case | Author | Year of publication | Age | Region | Symptoms |
|---|---|---|---|---|---|
| 1 | Chan, et al. [3] | 1992 | 10 | Left inguinal | Left inguinal mass |
| 2 | Chan, et al. [3] | 1992 | 50 | Right inguinal | Right inguinal mass |
| 3 | Allen, et al. [17] | 1993 | 67 | Right femoral | Right femoral mass |
| 4 | Laeng, et al. [5] | 1996 | 17 | Right cervical | Right cervical mass |
| 5 | Magro, et al. [18] | 1997 | 34 | Left inguinal | Left inguinal mass |
| 6 | Süllü, et al. [19] | 2005 | 33 | Right inguinal | Right inguinal mass |
| 7 | Piedimonte, et al. [20] | 2006 | 34 | Left inguinal | Left inguinal mass |
| 8 | Adaime, et al. [21] | 2009 | 74 | Right inguinal | Right inguinal mass |
| 9 | Bourgeois, et al. [22] | 2009 | 15 | Left inguinal | Left inguinal mass |
| 10 | Barzilai, et al. [4] | 2009 | 51 | Left submandibular | Left submandibular mass |
| 11 | Kim, et al. [6] | 2011 | 37 | Right popliteal | Swelling and pain in the right knee |
| 12 | Dzombeta, et al. [9] | 2012 | 67 | Right inguinal | Right inguinal mass |
| 13 | Catania, et al. [11] | 2012 | 8 months | Midline cervical | Midline cervical mass |
| 14 | Ding, et al. [23] | 2014 | N/A | N/A | N/A |
| 15 | Lee, et al. [24] | 2015 | 89 | Right inguinal | Right inguinal mass |
| 16 | Plantinga, et al. [16] | 2015 | 45 | Pelvic lymph nodes | N/A |
| 17 | Arava, et al. [25] | 2016 | 70 | Right inguinal | Right inguinal mass |
| 18 | Arava, et al. [25] | 2016 | 22 | Right inguinal | Right inguinal mass |
| 19 | Arava, et al. [25] | 2016 | 24 | Right inguinal | Right inguinal mass |
| 20 | Moh, et al. [26] | 2017 | N/A | N/A | N/A |
| 21 | Moh, et al. [26] | 2017 | N/A | N/A | N/A |
| 22 | Moh, et al. [26] | 2017 | N/A | N/A | N/A |
| 23 | Moh, et al. [26] | 2017 | N/A | N/A | N/A |
| 24 | Moh, et al. [26] | 2017 | N/A | N/A | N/A |
| 25 | Moh, et al. [26] | 2017 | N/A | N/A | N/A |
| 26 | Moh, et al. [26] | 2017 | N/A | N/A | N/A |
| 27 | Moh, et al. [26] | 2017 | N/A | N/A | N/A |
| 28 | Woolley, et al. [27] | 2019 | 59 | Left inguinal | Pain |
| 29 | Pyakurel, et al. [28] | 2019 | 12 | Right inguinal | Right inguinal mass |
| 30 | Xu, et al. [29] | 2019 | 57 | Left inguinal | Left inguinal mass |
| 31 | Present case | 2021 | 37 | Paraaortic and pelvic lymph nodes | Weight loss, pain |
Angiomyomatous hamartoma of the lymph node can affect people at any age but is considered to most commonly affect people in their 60s [26]. The youngest patient reported was 8 months old with an enlarged cervical lymph node [12]. The disease usually persists for years and does not recur after resection of the affected lymph node. Only one exception of recurrence has been described in the literature – pain in the left inguinal area confirmed a recurrence 14 years after AMH-LN excision [27]. The patient was treated conservatively. The optimal treatment of AMH-LN is total resection of all enlarged lymph nodes.
The differential diagnosis of AMH includes lymphangiomyomatosis, which, unlike AMH, involves primarily thoracic and intra-abdominal lymph nodes: nodal leiomyomatosis with less pronounced vascular proliferation and angiomyolipoma of the lymph node. The latter is composed of the same tissues as in AMH, but the smooth muscle component shows increased cellularity, polymorphism and increased mitotic activity, as well as a typical immune profile with coexpression of melanocyte markers and estrogen, which were negative in our case.
In our case, the disease was manifested by weight loss and moderate pelvic pain with no evidence of inflammatory disease. The process involved pelvic and paraaortic nodes, which is not typical of AMH-LN. We did not perform a total resection of all enlarged lymph nodes during the surgery as we did not have a definitive diagnosis. The cryosection material did not provide information about the process. This forced us to stop the surgical intervention and wait for the permanent preparation.











