Introduction
Ovarian malignancies are India’s second most common gynecological malignancy next to cervical malignancy. A varied and nonspecific presentation and a lack of screening make early diagnosis difficult. Most ovarian malignancies are often diagnosed in advanced stages. Mature cystic teratoma (MCT) of the ovary accounts for 10–20% of all ovarian neoplasms and is most commonly seen in women of reproductive age group and less commonly in postmenopausal women [1]. Mature cystic teratoma is a germ cell tumor of the ovary and histologically consists of tissues arising from endoderm, mesoderm, and ectoderm. A malignant tumor transformation is a rare event that accounts for only 1–2% of cases, with squamous cell carcinoma (SCC) accounting for up to 80% of all cases [1, 2]. It is derived from the ectoderm component of mature teratoma of the ovary. Pure primary squamous cell carcinoma, occurring de novo, is rare. Patients may present with abdominal pain, distension of the abdomen, or mass per abdomen. Either way, as only parts of the lesion are composed of malignant transformation, preoperative diagnosis of the malignancy is difficult. We report here a case of SCC in a postmenopausal woman associated with a large ovarian dermoid cyst.
Case report
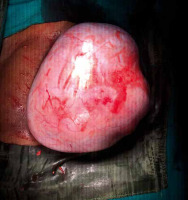
Mrs. X, a 65-year-old P4L4 woman, presented to us with complaints of intermittent lower abdominal pain for 6 months. An abdominopelvic mass corresponding to a 24 weeks gravid uterus was felt on examination. The mass was firm-cystic, had regular margins, and was mobile sideways. The patient was evaluated and was found to have hypothyroidism. We did a pap smear as she had not undergone any prior cervical cancer screening over the last 15 years. Her pap smear revealed a low-grade squamous intraepithelial lesion. Ultrasonography revealed a well-defined heterogenous lesion measuring 10.5 × 19.0 × 14.1 cm with typical features of a dermoid cyst likely arising from the right ovary (Fig. 1). Contrast enhanced computed tomography of the abdomen and pelvis confirmed the ultrasound finding as an ovarian dermoid cyst probably arising from the right ovary measuring 14.7 × 16.3 × 18.3 cm. No metastatic deposits were detected anywhere in the abdomen and pelvis, nor were there any lymph node enlargements. Her tumor marker CA125 level was normal. Intraoperatively a dermoid cyst of 20 × 15 cm was seen arising from the right ovary; around 15 ml of blood-stained ascitic fluid was present, which was sent for cytology, and no peritoneal deposit was noted anywhere in the peritoneal cavity (Fig. 2). She underwent a total abdominal hysterectomy with bilateral salpingo-oophorectomy. The postoperative period was uneventful, and the patient was discharged. On follow-up, the histopathology report revealed a right ovarian dermoid cyst with well-differentiated SCC confined to the cyst wall; there was no evidence of malignancy elsewhere, including the cervix (Figs. 3, 4). Ascitic fluid was also free of malignant cells, and the disease was at stage Ia. The patient did not receive any adjuvant chemotherapy and was followed up with clinical examination postoperatively for 1 year, and there was no evidence of any relapse clinically. This case is being reported with the consent of the patient.
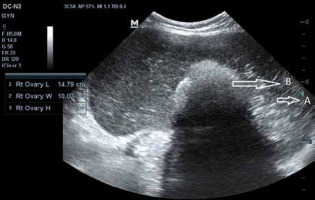
Fig. 1
Transabdominal ultrasound image showing huge cyst with (A) multiple thin, echogenic bands caused by the hair in the cyst cavity the dash-dot pattern (dermoid mesh), and (B) mural hyperechoic Rokitansky nodule (dermoid plug), likely dermoid cyst
Discussion
Although benign MCT is a tumor of the reproductive age group and a malignant transformation is rare, it often occurs in postmenopausal women. The most common subtype is SCC. Squamous cell carcinoma occurring de novo is even rarer. Malignant transformation is derived from the ectoderm component of mature teratoma of the ovary.
A possible causal relationship between primary ovarian SCC and human papillomavirus (HPV) has been explored in recent times [3, 4]. Human papillomavirus is known to cause invasive SCC of the cervix. The incidence of cervix SCC has come down due to increased availability and prompt screening with a pap smear, HPV tests, colposcopy, etc. However, there has been a recent rise in cases of cervical adenocarcinoma. A pap smear is primarily a screening test for SCC of the cervix. The presence of exfoliated endometrial cells can be normal during menstruation and in the proliferative phase of the menstrual cycle in reproductive age. But the presence of these cells in postmenopausal women requires further evaluation to rule out endometrial cancer or cervical adenocarcinoma. All women with atypical endometrial cells on Pap tests need endocervical curettage and endometrial sampling to rule out malignancy from an endometrial cavity or endocervical canal [5]. It is also important to differentiate adenocarcinoma of the cervix from that of the endometrium as they may have similar presentations, but the management is different.
Squamous cell carcinoma, either primary or secondary to malignant transformation, may present with abdominal pain, distension of the abdomen, abdominal mass, etc. There may also be associated pressure symptoms. As with any such clinical presentation, further workup in the form of ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI) may be warranted on a case-to-case basis. Not only does it help in knowing the origin and nature of the tumor, but also it differentiates it from uterine tumors. It will also help to differentiate a probable benign lesion from a malignant one. In the case of uterine tumors at an older age, postmenopausal bleeding is the most common symptom at presentation; no matter how minimal or nonpersistent, it needs evaluation. Transvaginal sonography with color Doppler is an efficient and acceptable non-invasive method for the early detection of endometrial pathologies with a sensitivity of 97% and a specificity of 74% [6]. On detection of suspected endometrial abnormalities, the next step is to evaluate with hysteroscopic directed endometrial biopsy. It has 100% sensitivity and 97% specificity for the detection of endometrial cancer [6]. The rare gynecologic malignancy uterine sarcoma is diagnosed by the history of rapid growth, postmenopausal bleeding, and ultrasonographic findings of solid masses in the uterus with heterogeneous echogenicity and well-enhanced vascularity. Sometimes, very rarely, it can be diagnosed in endometrial biopsy when it spreads to the inner lining of the uterus. Evaluation of huge masses is difficult by ultrasound; the most preferred techniques are CT and MRI. This will confirm the diagnosis, differentiate benign from malignant masses, and also provides information regarding the extension and metastasis of malignant masses [7]. In our case, radiologically the appearance was identical to that often seen in cases of benign mature ovarian teratoma. As only parts of the lesion are composed of malignant tissue and symptoms are primarily nonspecific, it is difficult to diagnose the malignant transformation of a teratoma preoperatively unless invasion into adjacent structures is present. However, in the absence of evident tumor invasion or metastases, it may be challenging to preoperatively identify an aggressive component in ovarian teratomas due to their heterogeneity.
Various tumor markers have proven diagnostic and prognostic roles in different gynecological malignancies. A raised level of cancer antigen 125 (CA 125) ≥ 35 IU/ml supports the diagnosis of serous epithelial ovarian cancers. It is a strong prognostic factor for the overall survival and progression-free survival of ovarian tumors [7]. Carbohydrate antigen 19-9 (C19.9) is found raised in mucinous epithelial ovarian cancers and also in malignant hepatobiliary and pancreatic conditions. Carcinoembryonic antigen (CEA) levels may also be raised in epithelial ovarian malignancies. As of yet, no single tumor marker is thought to be best in the diagnosis of SCC in the ovary, but CEA appears to be the best screening marker for SCC arising from mature cystic teratoma, better than CA125 or CA 19.9, but this requires further research [8, 9]. Overall, elderly age at presentation, huge tumor size, and large solid components, especially if irregular in the tumor, are important predictive factors for malignant transformation [8].
There is no specific treatment protocol for SCC in the ovary. However, a mixture of surgery plus chemotherapy and radiotherapy may be tried depending on the stage, grade, and parity of the tumor. Unilateral oophorectomy is performed in a nulliparous or young woman where fertility is an issue with stage IA disease. In postmenopausal women, the treatment of choice would be a total abdominal hysterectomy with bilateral oophorectomy [10]. No consensus or standard guidelines are available regarding the optimum outcome of post-operative adjuvant management of SCC arising from MCT because of a relatively little known entity and limited studies. A systematic review carried out by Hackethal et al. stressed a high survival rate associated with complete resection of the tumor followed by adjuvant chemotherapy based on alkylating agents in advanced disease. The study showed a higher overall survival rate of 57.1 months when alkylating agents were administered compared to 25.2 months for those who received non-alkylating regimens [11]. Although the prognosis in the case of malignant transformation is poor, the earlier stage of the disease limited to the ovary shows a better prognosis compared to the advanced stage [12]. Squamous cell carcinoma arising from MCT poses diagnostic as well as therapeutic challenges, and more research is needed to reach a consensus for the treatment protocol.
Conclusions
Squamous cell carcinoma in the ovary is rare. It occurs primarily associated with a dermoid cyst or endometriosis and rarely arises de novo. Preoperative diagnosis is difficult and lacks specific screening markers or a treatment protocol, so more cases must be studied to reach a consensus for treating such cases. Considering the scarcity of case reports and studies about SCC arising from MCT, every experience with malignant transformation of MCT should be reported for a better understanding of the disease presentation and management.