Oesophageal cancer (OC) is the seventh most common cancer worldwide and the sixth most common cause of cancer-related death [1]. Trends in OC vary greatly; squamous cell carcinoma (SCC) is the most common histological type in Eastern Asia, while in Western countries, studies show an increasing trend towards adenocarcinoma, probably due to the higher prevalence of the main risk factors: obesity and Barrett’s oesophagus. On the other hand, renal cell cancer (RCC) is the ninth most common cancer worldwide [1] and in many cases is detected as an incidental finding during investigation for non-related diseases. Even though OC is well-recognised for its frequent association with other primary malignancies in Eastern countries [2–5], adenocarcinoma of the gastroesophageal junction (GEJ) is rarely correlated with other primary synchronous tumours in Western countries. The frequency of multiple primary malignancies in the same or different organ system/s ranges from 2% to 17% [2–5].The most common sites of other primary malignancies associated with EC are head and neck, lungs, and stomach; a fact that can be easily explained by the simultaneous exposure in the same aetiologic carcinogenic agents (i.e. tobacco and alcohol) [6]. However, only few cases of synchronous OC and RCC have been reported in the literature to date [7–10], suggesting that this is an extremely rare clinical scenario with a lack of existing guidelines for the appropriate therapeutic management. Taking into account the postoperative morbidity of oesophageal surgery, the decision for the treatment of a patient with synchronous renal cancer and gastroesophageal junction cancer is of paramount importance, even more if there is a plan for radical nephrectomy, which could compromise patient’s renal function. Herein, we present a case of synchronous gastroesophageal junction adenocarcinoma and incidentally discovered clear cell renal cell carcinoma that was successfully treated in our department with a one-stage operative procedure.
A 51-year-old male Caucasian patient was referred to our department complaining of progressively worsening epigastric pain as well as dysphagia of recent onset. He had insignificant past medical history, while no history of tobacco use, or excessive alcohol ingestion was reported. Physical examination was unremarkable. Haematological examination and blood chemistry were within normal limits. The patient subsequently underwent an oesophagogastroscopy, which revealed a 4-cm lesion, located in the GEJ, causing partial obstruction of the oesophageal lumen. The histopathological examination was indicative of an adenocarcinoma of the GEJ. Contrast-enhanced chest and abdominal computed tomography (CT) was performed, which revealed a bulky tumour of the GEJ, with no evidence of lung, liver, or regional lymph node metastases (Figure 1). Also, the CT scan revealed an incidentally discovered heterogeneous mass of the upper pole of the left kidney approximately 2.5 cm, with no evidence of invasion of the renal capsule (Figures 1 C, D). The renal lesion was also detected by magnetic resonance imaging (MRI) as an enhanced mass of the superior pole of the left kidney in axial T1-weighted gradient echo images after gadolinium administration (Figure 1 D). The multidisciplinary team suggested a one-stage operative procedure, taking into consideration the patient’s age, his good general condition, as well as an initially presumed clinical stage of gastroesophageal junction cancer of T2N0, while kidney tumour was clinically staged as T1N0. Under general anaesthesia, with the patient in the supine position, exploratory laparotomy was carried out through an upper midline incision. Intraoperative inspection was negative for distant metastases. However, there was bulky lymph node disease present along celiac axis, along with the presence of a bulky tumour adjacent to, but not infiltrating, nearby structures. After abdominal exploration, the stomach was mobilised on the right gastric and right gastroepiploic arteries. The left gastric artery was divided at its origin and all lymph nodes were harvested with the vessel. Lymph nodes extending from the right gastric vessel through the base of the celiac axis and nodes superior to the proximal portion of the splenic artery and those along the lesser curvature and upper greater curve of the stomach were also included in the resection (stations 1–3, 4a, 7–9, and 11p). Subsequently, a typical radical left nephrectomy with limited lymph node dissection due to the early stage of kidney malignancy was performed and a gastric conduit was constructed using a stapling device (Endo-GIA II; Covidien, Norwalk, CT). A right posterolateral thoracotomy through the fifth intercostal space was then carried out, followed by resection of the distal two thirds of the thoracic oesophagus along with lymph node dissection of all paraesophageal and subcarinal lymph nodes (including, station 7 and level 8R, 8L, and 9), and the specimen was resected. Gastrointestinal continuity was reestablished through an esophagogastric end-to-side hand-sewn anastomosis in the upper mediastinum. Postoperatively, the patient was admitted to the intensive care unit for 24-hour close monitoring. The patient’s postoperative course was uneventful, and he was discharged on the ninth postoperative day, while the patient’s renal function and urine output were maintained within normal values at all times. Initiation of oral diet was smooth, and removal of drains and chest tube followed with no significant complications based on Clavien Dindo classification. The histopathologic study revealed a poorly cohesive carcinoma of the gastroesophageal junction, diffuse-type according to Lauren classification, with signet-ring cell features (Figure 2 A). The tumour was 4.5 × 4.3 cm in size, invading the muscular wall and extended to the adjacent periesophageal tissues. Sixty-eight lymph nodes were harvested, 38 of which were positive for malignancy. The final pathological staging was pT3N3M0, according to WHO 2017 classification (eighth Edition). Interestingly, in agreement with current literature reporting that more than 50% of patients with early oesophageal cancer are under-staged while more than 50% of them suffer from node-positive disease, histopathology showed that we also significantly under-staged gastroesophageal tumour in our case [11]. If an advanced nature of the disease was apparent preoperatively, the multidisciplinary team would have suggested initially following a neoadjuvant treatment pathway, instead. The kidney lesion was reported as a clear cell renal cell carcinoma, 2.7 cm in maximum diameter, nuclear grade II according to International Society of Urologic Pathologists (ISUP) 2016 classification, located at the superior pole of the kidney, which did not invade the renal capsule nor the renal pelvis (Figure 2 B). The final pathological staging was pT1a according to WHO 2017 classification (eighth Edition). The patient was offered 6 cycles of adjuvant chemotherapy with oxaliplatin and Farmorubicin, and remains disease-free 9 months postoperatively (Figure 3). Along with improvements in the accuracy of diagnostic imaging studies, the detection of incidentally synchronous primary tumours in patients with oesophageal cancer and gastroesophageal junction cancer is constantly increasing. According to Warren and Gates [12], the criteria for the diagnosis of double primary malignancies are the histological confirmation of malignancy in both – index and secondary – tumours, a minimum distance of 2 cm of normal mucosa between the tumours, and the exclusion of the possibility of one lesion being a metastasis of the other. Oesophageal cancer is frequently associated with other synchronous primary malignancies (OPM) in countries with high prevalence of OC, such as countries of Eastern Asia [2–5]. According to the Japanese Oesophageal Society, the incidence rate of synchronous OPM is 10.1–12.4%. To date, the risk of developing simultaneous primary malignancy in patients with EC in Western countries is not well-documented, mainly due to the low incidence of OC in the West.
Figure 1
A – CT image depicting gastroesophageal junction tumour. B – CT image depicting gastroesophageal junction tumour along with a great proportion of the liver parenchyma with no visible metastatic deposits. C – MDCT image, slicing through kidneys and oesophagus depicts diffuse wall thickening of the distal one-third of the oesophagus and gastroesophageal junction and solid hypodense mass at the left kidney upper pole. D – Axial T1-weighted gradient echo MRI image after gadolinium administration shows a solid contrast-enhanced mass at the left kidney upper pole
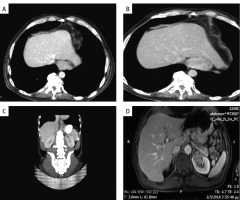
Figure 2
A – Diffuse type carcinoma of the gastroesophageal junction, with signet-ring cell features, (H-E, 100×X). B – Grade 2 clear cell renal cell carcinoma (H-E, 100×)
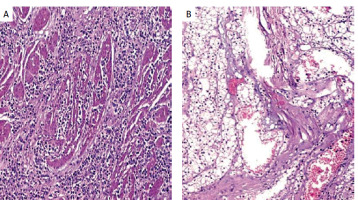
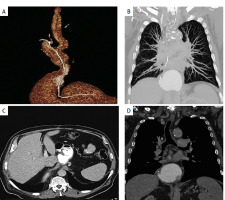
Figure 3
A – MDCT reconstructed image, depicting esophagogastric anastomosis. B – CT image depicting lung parenchyma in follow-up visit of the patient. C – CT image depicting liver parenchyma with no suspicious lesions for metastasis as well esophagogastric anastomosis. D – CT image depicting mediastinum with no suspicious lesions for metastasis in follow-up visit of the patient
Currently, there is no universally accepted diagnostic modality with overall oncologic accuracy, and there are no established diagnostic protocols for identifying synchronous multiple primary malignancies (MPMs). The selection of imaging technique, such as computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and ultrasound (US), depends on factors including tumour characteristics and anatomical location [13]. Among these modalities, contrast-enhanced multidetector CT (MDCT) is widely utilised and recommended for cancer staging due to its ability to provide a comprehensive evaluation of the entire body in a remarkably short period. MDCT, employing volumetric acquisition, facilitates multiplanar reconstruction of relevant tissues and organs, enabling detection of both primary and secondary tumours in nearby or distant organs. However, CT’s sensitivity in identifying additional tumours, particularly those resembling benign abnormalities (e.g. cutaneous melanoma, small thyroid tumours, or prostate carcinoma), is relatively modest (85–90%) despite its effectiveness in assessing tumour extension [13]. To enhance the detection of metastatic disease and MPMs, administration of intravenous contrast material with a multiphasic approach is indispensable. Techniques such as PET, which provide non-invasive insights into cellular metabolism, are employed to identify unexpected tumour sites by detecting elevated uptake of radioactive tracers. In clinical practice, the most commonly utilised radiotracer is 18F-labeled fluorodeoxyglucose (FDG). All the aforementioned imaging techniques are of paramount importance when used preoperatively in such cases with multiple primary malignancies [13]. Recent studies have assessed the risk of simultaneous development of OPM in EC patients; however, data on synchronous OPM in OC patients in the West are limited as the majority of these studies originate from eastern countries (Table I). Papaconstantinou et al. in a systematic review published in 2019 [14] reported 5 patients from 23 eligible studies suffering concomitantly from oesophageal and renal cancer. In an Asian study, Lee et al. [6] retrospectively reviewed 622 OC patients and reported that 90 (14.5%) OC patients presented with other primary cancers, whereas 43 presented with synchronous primary malignancies. Overall, the 3 leading sites of second primary malignancy were stomach (n = 36, 37.5%), head and neck (n = 18, 18.8%), and lungs (n = 18, 18.8%). Interestingly, 87 out of 90 OC patients who presented with second primary malignancy, were diagnosed with oesophageal SCC. None of the cases of multiple primary malignancies along with OC included in this study involved renal cell cancer. In a recent retrospective study [15], Lee at al., stated that out of 317 patients with oesophageal SCC, synchronous primary malignancies were identified in 21 (6.6%) patients, while head and neck and lung cancers were the most common SPC (40.9%), followed by gastric cancer (18.2%). Oesophageal squamous cell carcinoma has been reported to be frequently associated with other tobacco-related cancers such as those of the oropharynx, larynx, and lung. Oesophageal adenocarcinomas are most commonly associated with tumours of the stomach, oropharynx, pancreas, kidney, colon, and rectum [16, 17]. However, the simultaneous detection of oesophageal and renal cell adenocarcinoma is relatively rare, with only few cases reported in the literature. Kobayashi et al. [8] reported an occurrence rate of 1.6% of synchronous development of oesophageal and renal cancer, while de Hingh et al. [18] reported an estimated incidence of incidentally identified renal cell cancer (RCC) in OC patients of 2.1%. Moreover, Sato et al. [19], evaluated the incidence of multiple primary malignancies involving RCC in a Japanese population and stated that out of 319 patients with RCC, 38 (12%) presented with at least one other malignancy, while 19 (6%) cases were of synchronous detection. Only 2 cases of simultaneous development of OC and RCC were reported in this study. In addition, the retrospective study by van Westreenen et al. included 366 EC patients from the Netherlands who underwent preoperative 18F-FDG PET for initial staging [20]. Synchronous primary malignancies were identified in 20 (5.5%) patients, whereas in 14 cases the primary OC was adenocarcinoma. Concerning the synchronous OPM, 11 cases included colon and rectum, 5 the kidney, 2 cases included the thyroid gland, and one was located in the lung. Notably, all the cases of synchronous renal neoplasms were histopathologically identified as renal clear cell carcinoma, while in 4 cases the primary OC was adenocarcinoma. The detection of multiple primary malignancies raises concerns that must be addressed. Principally, in cases of localised disease, the optimal therapeutic option may be surgery for both malignancies with either a one-stage or a two-stage procedure. However, in cases of advanced disease requiring neoadjuvant therapy, the question is whether the same chemotherapeutic agents are efficient for both malignancies, and if not, which malignancy needs to be given therapeutic priority. Additionally, when dealing with multiple primary tumours, it is of great importance to address which malignancy is associated with poorer prognosis, to achieve the optimal therapeutic outcome. Concerning the cases of synchronous oesophageal and renal cancer, it is well-established that the overall survival in patients with RCC is higher than that in the OC patients [1]. Therefore, the treatment of RCC does not take precedence over that of OC. Nonetheless, the therapeutic strategy needs to be in line with the clinical stage of each malignancy, i.e. T-stage, grade, and nodal disease. A tailor-made approach including high-quality surgery and an optimal choice of chemotherapy and radiotherapy regimens according to the characteristics of both cancers could achieve a potential cure or best possible survival benefit in cases of multiple primary malignancies. In our case, in line with all previous cases reported, gastroesophageal junction cancer was detected prior to RCC, which was incidentally detected on preoperative CT. In the literature, besides abdominal CT, synchronous RCC can be diagnosed by ultrasonography, MRI, or 18F-FDG PET and is mainly asymptomatic. The detection of synchronous RCC during the pre-operative assessment of our patient invoked a serious therapeutic dilemma regarding the optimal treatment strategy. Considering the excellent clinical condition of our patient and the early clinical stage of the renal and gastroesophageal junction tumour, we performed a one-stage procedure, resecting both lesions, with an uneventful postoperative course. Management of both neoplasms via a one-stage procedure probably offers some advantages such as a decreased total time for recovery and reduced total hospital stay. However, the two-stage procedure is probably associated with lower morbidity rates for each stage, thus enabling the performance of 2 major surgical procedures. All in all, we acknowledge that there is considerable debate among researchers as to the timing of each procedure. However, evidence-based arguments about whether to perform a one-stage operation or a staged operation are not available. Therefore, a one-stage procedure with curative intent may be feasible in selected patients with simultaneous oesophageal and renal cancer. However, further studies are required to determine the benefit of these major procedures in terms of survival outcomes. The rate of incidental detection of synchronous neoplasms in OC patients is increasing. However, data on the incidence and distribution of simultaneous OPM in OC patients are limited in the West. Consequently, guidelines for treatment are not yet available. Our results on managing a case of simultaneous detection of OC and RCC, suggest that a one-stage operation is feasible if proper care is given to patient selection. Therefore, future research should be conducted including prospective data to test new therapeutic strategies.
Table I
Characteristics of patients undergoing simultaneous resection of oesophageal and kidney neoplasms
| Author | Number of cases | Age | Oesophageal type of surgery | Renal surgery |
|---|---|---|---|---|
| Kobayashi et al. [8] | n = 2 | 61 | TTO (n = 2) | Nephrectomy (n = 2) |
| Liano et al. [7] | n = 1 | 64 | TTO (n = 1) | Nephrectomy (n = 1) |
| De Hingh et al. [18] | n = 1 | 69 | THO (n = 1) | Nephrectomy (n = 1) |
| Vilcea et al. [9] | n = 1 | 56 | TTO (n = 1) | Nephrectomy (n = 1) |










