Introduction
Since the initial outbreak of coronavirus disease 2019 (COVID-19) – which is brought on by the SARS-CoV-2 coronavirus – in Wuhan, China, the virus has rapidly spread throughout the world. More than 760 million SARS-CoV-2 infections and about 6.8 million fatal cases have been documented as of April, 4, 2023 globally [1]. After the first COVID-19 case was discovered, the virus rapidly spread among people worldwide [2]. The primary mode of transmission is through airborne droplets generated when an infected person coughs or sneezes. These droplets can contain the virus and infect others who come into close contact with them. The contagious nature of the virus and its ability to spread through respiratory droplets contributed to the global spread of COVID-19 [3]. The COVID-19 patients noted a number of significant symptoms, including fatigue, myalgia, headache, fever, loss of smell, and dry cough [4]. Patients infected with SARS-CoV-2 exhibit acute serological responses, indicating a high risk of infection. This ongoing threat poses a significant challenge to global public health [5]. Vaccination is widely recognized as the most effective approach to combat COVID-19. COVID-19 vaccines are being offered and administered in various locations worldwide. These vaccines play a crucial role in preventing severe illness, reducing hospitalizations, and minimizing the transmission of the virus. Vaccination efforts are an essential part of global public health strategies to control the spread of COVID-19 [6]. The vector-based ChAdOx-1 nCOV-19 vaccine (Vaxzevria, Oxford/AstraZeneca, UK), also known as AZE, was recommended for individuals over 60 and at high risk of contracting the disease due to its age-dependent safety profile. This vaccine is one of the authorized COVID-19 vaccines that are currently offered in Germany [7]; however, it is no longer advised because of uncommon but serious thrombotic effects [4]. The BNT162b2 vaccine (Comirnaty, BioNTech/Pfizer, Mainz, Germany), abbreviated as BNT, is administered to people aged 5 and up. BNT162b2 and mRNA-1273 are both mRNA-based vaccines. Furthermore, the mRNA-1273 (Spikevax, Moderna, Cambridge, MA, USA) vaccine, also known as MOD, is considered effective and safe, particularly for people aged 30 and up [8]. Individuals who have previously contracted the coronavirus will also be given the vaccine. At least two doses of the same vaccine are required for complete immunization [9]. Due to vaccine availability and evolving knowledge about COVID-19, a combination of vaccines has been accepted as a complete immunization strategy. As antibody titers can decrease over time, a booster vaccination is recommended around 6 months after the initial vaccination to maintain continued protection against SARS-CoV-2. This approach aims to enhance and prolong immunity against the virus, taking into account the dynamic nature of the pandemic and the need for long-term protection [10]. Pfizer-BioNTech’s COVID-19 vaccine is an effective means of combating the pandemic. Concerning the efficacy and severity of homologous vaccination, it was found that those infected with SARS-CoV-2 who had been vaccinated with double doses (fully vaccinated) of vaccines had a lower risk of hospitalization, emphasizing the importance of launching the booster doses [11–13].
Individuals infected with SARS-CoV-2 are expected to produce both N-specific antibodies and S-specific antibodies, whereas mRNA vaccines aim to produce only S-specific antibodies. Furthermore, mRNA vaccinations demonstrated booster effects even in individuals who had previously received COVID-19 [14, 15]. Sometimes after COVID-19 vaccination, the process of building immunity can cause adverse side effects (SE) and interactions. Acute SE following a COVID-19 vaccine can be divided into two categories: local and systemic. Swelling, redness, pain at the injection site, and skin sensitivity are examples of local side effects. Fatigue, diarrhea, nausea, muscle pain, joint pain, headache, shivering, and fever are examples of systemic side effects [16]. Most reported SE resolve within a few days; however, severe SE such as anaphylaxis and thrombotic events have been reported on rare occasions [17, 18]. In contrast, messenger RNA (mRNA) vaccines against SARS-CoV-2 show significant promise in limiting the spread of infection. These vaccines utilize mRNA technology to instruct cells to produce a harmless piece of the virus, triggering an immune response. mRNA vaccines have demonstrated high efficacy in preventing COVID-19 and reducing the severity of the disease. Their development and deployment offer a powerful tool in curbing the spread of SARS-CoV-2 and mitigating the impact of the pandemic [19].
In the current study, we enrolled a large number of cases who received the BNT162b2 mRNA vaccine. We systematically evaluated short-term reported vaccine SE after the Dose 1 (D1) and Dose 2 (D2) vaccinations. Moreover, clinical markers were evaluated, including IgG (S-RBD) antibody titers, white blood cell (WBC) count, CRP and D-dimer in terms of sex, age and comorbidities of participants in 2022.
Material and methods
Study design and sample setting
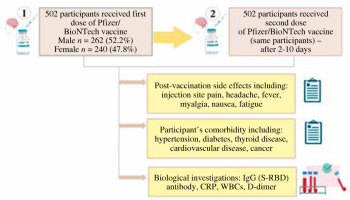
In this study conducted between March 2022 and September 2022, 502 participants were enrolled at the Sherwana Medical Centre for COVID-19 vaccination in Kalar, Sulaymaniyah province, Iraq. The participants received two doses of the Pfizer/BioNTech vaccine (BNT162b). The study involved two categories of inquiries: The first was a questionnaire: Participants were asked to provide background data such as gender, age, and any short-term SE experienced after receiving the vaccine. Additionally, the survey assessed the occurrence of morbidity for each participant. Secondly, serological investigations were performed: The second set of inquiries focused on serological investigations, likely involving the analysis of blood samples to evaluate the immune response generated by the vaccine (Fig. 1).
These inquiries aimed to gather data on the participants’ demographic information, vaccine side effects, and the effectiveness of the Pfizer/BioNTech vaccine in terms of serological responses. The study sought to contribute to the understanding of vaccine outcomes and COVID-19 vaccination efforts in the region.
Questionnaire development
The study’s objectives were described in an introductory letter that was included with the questionnaire, which was distributed and filled out by direct face-to-face interviews with participants and took nearly 15 minutes to complete. The questionnaire version consisted of 13 questions divided into two sections. Hence, we report the post-vaccination group SE in terms of sex, age and comorbidity in section 1. The SE information of the vaccination was obtained by questionnaire after D1 and D2, including: injection site pain, headache, fever, myalgia, nausea, and fatigue, and additionally, participant’s comorbidity including: hypertension, diabetes, thyroid disease, cardiovascular disease, and cancer (Supplementary Table 1, Fig. 1).
Biological markers
Serum samples were collected from participants after 3-10 days for both vaccination doses (recovery time average). In this study, various biological investigations were conducted, including the measurement of IgG antibodies targeting the SARS-CoV-2 spike receptor-binding domain (S-RBD), WBCs, C-reactive protein (CRP), and D-dimer levels. The levels of anti-nucleocapsid IgG antibodies, indicative of the immune response generated after infection, were determined using a commercially available SARS-CoV-2 IgG test kit (Pishtaz-TebDiagnostics, Tehran, Iran). This kit specifically assesses the antibodies developed in response to the SARS-CoV-2 virus, not the virus itself.
Pishtaz-TebDiagnostics, a reputable company based in Tehran, Iran, has developed multiple kits designed to detect IgG antibodies associated with SARS-CoV-2 infection. In this research, the kit utilized for the quantitative measurement of anti-nucleocapsid IgG antibodies was selected. The manufacturer’s predefined criteria were applied for interpretation: a result of 1.1 or higher was considered positive, results falling between 0.9 and 1.1 were deemed equivocal, and results less than 0.9 were categorized as negative. Hence, an M-Series hematology analyzer from SweLab coulter count for CBC was used (SeweLAb CBC Analyzer, Boule Medical AB, Stockholm, Sweden). A fully automated multi-parametric analyzer, the Cobase C111, was used to investigate the serum CR protein level (Roche Diagnostics, Mannheim, Germany). Fluorescence immunoassay ichroma II (Boditech Med Inc.) was used for the D-dimer Rapid Quantitative Test, with a normal value being < 500 ng/ml [20]. In this particular facility, IgG levels were documented for everyone who received the vaccine. However, in our research, we specifically included individuals who had negative IgG levels before vaccination.
Statistical analysis
Statistical analysis was carried out using two-way analysis. GraphPad Prism 9.3 was used to observe the statistically significant differences risk ratio between the control and test group for all parameters. In addition, the paired t-test was used to analyze parameters between first and second dose groups (NS = p ≥ 0.1234; significant: *p ≤ 0.0322, **p = 0.0021, ***p = 0.0002, ****p < 0.0001).
Ethical declaration
The study received approval and adhered to applicable guidelines and regulations throughout all its procedures. Also, we confirm that all experimental protocols were approved by the Ethics Licensing Committee of the Kalar Technical Institute at the Sulaimani Polytechnic University Committee (No. KTC04 on April 25, 2022). In addition, informed consent was obtained from all subjects or, if subjects were under 18, from a parent and/or legal guardian.
Results
Study cohort
Between March 2022 and September 2022, a total of 502 participants (male [M]: n = 262 (52.2%), female [F]: n = 240 (47.8%)) were included in the analysis. All of the participants included currently were receiving Pfizer-BioNTech vaccination (100%). The study groups were as follow: healthy = 258 (male: 127 and female: 117) and morbid = 244 (male: 135 and female: 123). (These data are summarized in supplementary material: Fig. 1 and Table 1) [21].
Participants who reported side effects
The adverse reactions and SE reported among participants who received COVID-19 vaccination are shown in Supplementary Table 1. The most common SE reported after D1 vaccinations by the study participants was ISP (45%), followed by equal proportions of headache and fever (40%), then nausea, myalgia and fatigue (11%, 8% and 4%, respectively). Likewise, the D2 side effects were as follows: ISP (66%), nausea (57%), headache (53%), myalgia (50%), fatigue (47%) and fever (41%) and the questionnaire encompassed inquiries about “additional adverse reactions [22]” with no instances of reported effects. Furthermore, participants who received two doses experienced significantly more side effects (p < 0.0001) (Fig. 2). Hence, we evaluated the correlation between healthy and comorbid participants with the severity of short-term SE. Importantly, the D2 vaccination between comorbid and healthy group participants had a significantly higher incidence with differences in D1 vaccination (p < 0.0001) (Fig. 2). No significant differences were reported in the D1 group, while in D2 vaccination more side effects were noted in comorbid than healthy participants (Significant: p < 0.0001) (Fig. 2).
Blood-type-related investigations
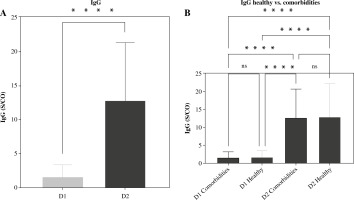
Interestingly, in the cohorts receiving both D1 and D2 vaccinations, there was no notable correlation found between the development of IgG antibodies and variables such as age, gender, and co-morbidity. However, significant distinctions were noted in IgG antibody generation across different genders, ages, and co-morbidity statuses within each D1 and D2 vaccination group (p < 0.0001) (Figs. 3-5, Supplementary Fig. 3). The current study revealed a significant difference in age between healthy and morbid participants (p = 0.0168) and showed no significant differences between M and F regarding health and morbidity in both vaccination doses (p > 0.4037). However, a significant difference was observed between M comorbid participants and healthy F (p = 0.0103) (Supplementary Fig. 2).
Fig. 3
The cumulative occurrences of IgG antibodies among study participants, categorized by gender and comorbidity status (X-axis), with IgG values indicated on the Y-axis. The chart provides insight into the prevalence or concentration of IgG antibodies following the initial (D1) and second (D2) Pfizer/BioNTech vaccine doses
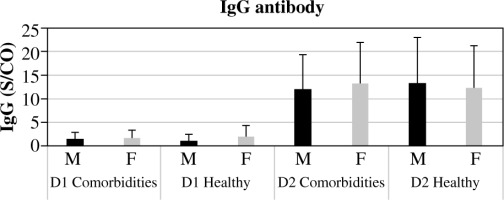
Fig. 4
The frequency of IgG antibodies (Y-axis) in study participants across different age groups (X-axis) following the first (D1) and second (D2) doses of the Pfizer/BioNTech vaccine. This chart offers insights into the prevalence or concentrations of IgG antibodies within distinct age categories, providing an understanding of the vaccine-induced immune response across diverse age groups
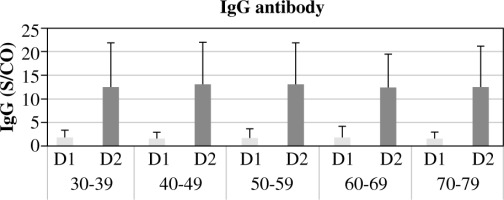
Fig. 5
The occurrence of IgG antibodies (Y-axis) in study participants, categorized by comorbidity across various age groups (X-axis), following the first (D1) and second (D2) doses of the Pfizer/BioNTech vaccine
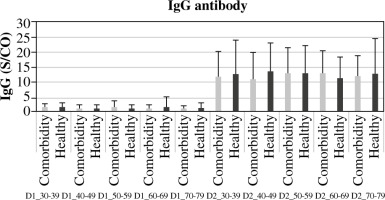
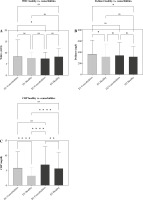
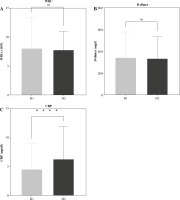
Comorbidities related to IgG antibody responses are reported in Supplementary Figure 3. A statistically significant (< 0.0001) difference was detected between D1 and D2 in the generation of IgG responses with all SE reported in this study concerning mean antibody level. Moreover, considering other biomarkers as explanatory variables, the evaluation of biological markers showed a significant difference in serum CRP level between participants who received D2 vaccination (p < 0.0001 for each) in comparison to participants with D1 vaccination. Concerning total WBC count and D-dimer level, no significant differences between D1 or D2 vaccinated participants were observed: p = 0.3995 and p = 0.3957 respectively (Fig. 6).
Fig. 6
Investigation of clinical indicators, namely A) white blood cell (WBC) counts, B) D-dimer, and C) C-reactive protein (CRP) levels. The graph illustrates variations in these indicators within the study cohort, with “ns” signifying no significant differences, and p ≤ 0.05 indicating significant differences
Regarding the chronic disease group, we found that the biological markers showed different values between healthy and morbid participants. Our data showed significant correlations between both doses in evaluation for IgG antibody between D1 healthy and D2 comorbid and healthy (p < 0.0001). Likewise, serum CRP level in D1 with groups, D1 healthy with D2 comorbid and healthy were significant (p < 0.0001 respectively) and a significant value (p = 0.0036) was obtained in D2 between the two groups. There was no significant correlation between IgG antibody and total WBCs in D1 or D2 for morbid and healthy participants, while D-dimer exhibited a significant difference within D1 (p = 0.0122) with no significant value in D2 (p = 0.4773) despite the significant difference between D1 comorbid and D2 healthy (p = 0.0122) (Fig. 7).
Discussion
Concerns about the possible dangers and risks of vaccination administration have been expressed by the public since vaccine manufacturing began. People’s trust in vaccinations varies greatly, depending on factors such as vaccine knowledge, chronic infection, experiences, religious or political beliefs, and socioeconomic status [23]. The results of our study confirmed that the adverse effects of D2 of an mRNA vaccination for the individuals were significantly greater than those of the D1 vaccination. This result is consistent with previous studies [4, 16]. This finding might be explained by the reaction of the immune system. Emerging real-world data show, for instance, that the immune response of individuals with past SARS-CoV-2 infection after a single dose of the vaccine is comparable to or greater than that of SARS-CoV-2-naive individuals following the second dose. Accordingly, in patients who have higher immunity to SARS-CoV-2, the subsequent dosing may result in the generation of cytokines that may have an inflammatory impact on muscles, blood vessels, and other tissues, and development of widespread side effects [24]. Previous research demonstrated that following the second dosage, headache and nausea were more common. In contrast, the percentage of fever remained stable [16]. The current study found that the D1 side effects of the COVID-19 immunization in people with comorbidities were not statistically significantly different when compared to those in healthy people, which is consistent with earlier studies [25]. Contrary to the findings of a Saudi Arabian study, more adverse events occurred in the comorbid patients after the second dosage compared to the healthy individuals [26].
The receptor binding domain (RBD) region in the spike protein of SARS-CoV-2 is a critical target for neutralizing antibodies [27]. So, in our study, we used an ELISA kit for the qualitative measurement of IgG to detect the spike-RBD (S-RBD) antibody that was produced by the immune cells in response to the S gene mRNA of the vaccine. Studies have shown that stronger S-RBD IgG binding leads to better protection and that larger titers of S-RBD IgG correlate positively with a decreased disease state and fewer breakthrough infections [27, 28]. Individuals vaccinated with mRNA who develop lower levels of anti-spike RBD IgG antibodies are more likely to get infected [29]. In this study, the rate of seroconversion increased significantly after D2 was administered (Supplementary Table 1). This finding is in line with the pharmacist’s recommendation of a two-dose vaccination schedule [30], which emphasizes the need for administering the full course of immunization. Older individuals with comorbidities have been excluded from Phase III of the efficacy trial, and there are few published effectiveness data for this population [31]. Many studies have reported consistent IgG and IgA levels after both rounds of vaccination, regardless of prior exposure. This finding implies that the antibody increments were comparable across the entire vaccinated group, regardless of their initial IgG levels before vaccination. Consequently, all participants achieved seroconversion after the second dose, demonstrating similar antibody levels [32, 33]. Another study reported that, within eight months after vaccination, both IgG and IgA responses diminished significantly, dropping by 2.4 times for IgG and 2.7 times for IgA. Despite this decline, no infections were recorded within the group. Following the booster shot, the entire cohort exhibited elevated IgG levels immediately, followed by a specific increase in IgA. Post-booster IgG levels surpassed those after the initial vaccination, while IgA was restored to its previous levels [34, 35].
In this study, healthy and comorbid individuals of young and older ages did not significantly differ in the rise of IgG after D2 (Fig. 3), in contrast to a previous study that found that people over 60 had considerably lower IgG titers after the second dose [36]. In addition, contrary to our findings, the male gender had lower IgG levels after vaccination [37]. It has been reported that people with healthy dietary habits showed greater IgG titers following the first and second doses of the COVID-19 vaccine than those with less healthy eating habits [38].
In this study there was no recorded leukopenia after vaccination, which is in line with a prior study [11]. However, our result is contrary to a study which observed an increased risk of leukopenia following the second dose of BNT162b2 [39]. In our study only the morbid participants showed a significant decrease in the WBC count, although this decline still fell within the normal range. Vaccine-induced thrombotic thrombocytopenia (VITT), which is a cerebral venous sinus thrombosis with thrombocytopenia, has been documented nearly exclusively after the Astra-Zeneca-Oxford and Johnson & Johnson adenoviral vaccines, and most often after the first dose of immunization [40]. In virtually all recorded occurrences of VITT, D-dimer levels are considerably elevated [41]. D-dimer testing is advised by international guidelines for VITT screening [42]. The British guidelines suggest that VITT is very improbable when D-dimer levels are below 2000 ng/ml [43]. In the current study, we measured the D-dimer levels for the possible occurrence of VITT following immunization. There was no significant link between the D-dimer levels of the comorbid and healthy participants after the second dosage, despite the fact that the comorbid subjects had much higher D-dimer levels than the healthy subjects following the D1 vaccine. The D-dimer level is always within the acceptable range, and no cases of VITT have been documented. This outcome is consistent with earlier research showing that since the launch of the vaccination from Pfizer-mRNA BioNTech, there have not been any published case reports [44]. CRP is a key pro-inflammatory marker that rises during infection and falls as the patient heals [45, 46].
It serves as an immune system and body biomarker, increasing after influenza and pneumococcal vaccinations [47]. CRP testing is a common aspect of cardiac therapy because of the link between raised levels of CRP and an increased risk of cardiovascular disease [48]. This study reported a modestly to moderately raised level after receiving the mRNA vaccine, suggesting a temporary inflammation. The morbid participant showed a significantly higher CRP level as compared to the healthy participant, which could be due to the higher baseline of their CRP baseline level due to their prior health condition. However, the healthy participant showed a significant rise in CRP level at D2 as compared to D1; at the same time, the morbid participant showed a non-significant rise. This may be due to the fact that healthy participants had the optimal immune response and the increase in CRP level is related to the acute phase reaction of the immune system. However, because of the previous chronic phase response of the morbid participant owing to the prior health condition, such a rise in the CRP level was not significantly different as compared to healthy participants [49]. To the best of our knowledge this is the first study tracking the level of CRP of morbid individuals as a response to subsequent COVID-19 vaccination.
Conclusions
Overall, it is worth mentioning that the Pfizer/Bio-NTech COVID-19 vaccine is typically safe and well tolerated with the majority of adverse effects being mild to moderate and disappearing within a few days, i.e., despite a brief period of post-vaccination side effects and infection, the newly established, genetically manipulated mRNA vaccine likely provides the best alternative for boosting immunity for pandemic control and protection of public health.