Introduction
Biliary atresia (BA), a multifaceted liver disease of complex pathogenesis, has devastating consequences for a child’s health with rapid progression to end-stage cirrhosis if not treated in a timely fashion [1, 2]. Biliary atresia results from an inflammatory and fibrosing obstruction of extrahepatic bile ducts [3]. Along with the general term ‘neonatal hepatitis’, BA is a leading cause of neonatal cholestasis; as a single disease, BA is the number one indication for pediatric liver transplantation worldwide [4, 5].
By the time of diagnosis, extrahepatic bile ducts are completely obstructed [4]. At the tissue level, segmental or wholesale loss of the epithelial lining of extrahepatic bile ducts exists, with extensive fibrosis and occasional foci of inflammation [2]. By contrast, intrahepatic bile ducts are typically hyperplastic, embedded in portal tracts that contain variable inflammation and fibrosis, and are surrounded by lobules with features of cholestasis and variable degrees of giant multinucleated hepatocytes [6, 7].
Biliary atresia is classified on anatomical bases, referring to the level and severity of the obstruction [8]. The more commonly used Japanese and Anglo-Saxon classification describes 3 main types [7]. In type I, atresia is limited to the common bile duct, and the gallbladder and hepatic ducts are patent (i.e., “distal” BA) [9]. In type II, atresia affects the hepatic duct, but the proximal intrahepatic ducts are patent (i.e., “proximal” BA) [10].
Type II is sub-grouped into type II a, where a patent gallbladder and patent common bile duct are present (sometimes with a cyst in the hilum, i.e., “cystic BA”), and type II b, where the gallbladder, the cystic duct and the common bile duct are also obliterated [11]. In type III, there is discontinuity of not only the right and left intrahepatic hepatic ducts, but also of the entire extrahepatic biliary tree (i.e., “complete” BA) [12]. The French classification is similar, but the designation of the above types II a and II b as types 2 and 3 results in a total of four types [10].
Despite the Kasai portoenterostomy which is performed at the time of diagnosis, BA usually leads to biliary cirrhosis and is the most common indication for pediatric liver transplantation [13]. There are various methods of diagnosis of fibrosis of the liver such as FibroScan, APRI index and FIB-4 [14].
Some blood markers have been proposed as predictors of hepatic fibrosis in liver diseases, including albumin, hyaluronic acid, aspartate aminotransferase (AST) to platelet ratio (APRI), AST to alanine aminotransferase ratio (AAR), Fibrosis-4 (FIB-4) index, and blood platelet count [15]. The immediate benefit of using non-invasive markers of fibrosis is avoiding the need for liver biopsy and possibly other more expensive procedures [16].
Liver biopsy has long been considered the gold standard for the assessment of liver fibrosis [17], but nowadays it has been almost entirely replaced by noninvasive methods that measure liver stiffness (LS), such as biochemical markers and scoring systems, such as the APRI score and FIB-4 scores [18]. The main advantage of biochemical noninvasive scores in evaluating liver fibrosis is that they are generally available at a low cost and are very simple to use. APRI and FIB-4 scores have been proved quite reliable for assessing liver fibrosis [19].
The aim of this study was to evaluate the validity of APRI and FIB-4 indices in prediction of fibrosis in patients with BA.
Material and methods
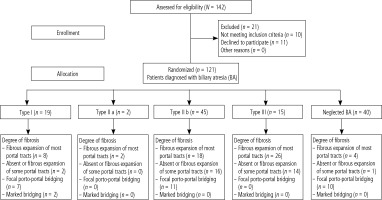
This was a cross sectional hospital-based study which was conducted on 121 children complaining of BA attending the National Liver Institute, Menoufia University, Shebin El Kom, Menoufia, Egypt, during the period from January 2022 to February 2023. All studied pediatric patients were divided into five groups: BA type I (n = 19), type II a (n = 2), type II b (n = 45), type III (n = 15) and neglected BA (n = 40).
Patients with BA had scores when they were diagnosed. Infants with BA and those without BA can be distinguished with accuracy using this scoring method. The intraoperative cholangiogram added further confirmation to the diagnosis [20].
Histopathological evaluation
All the liver wedge biopsies were processed, and formalin-fixed, paraffin-embedded (FFPE) tissue blocks were prepared (Fig. 1). Then 4-µm-thick hematoxylin and eosin (H and E) stained slides were obtained and stained with mason trichrome stain. All cases were histologically evaluated. Degree of liver fibrosis was determined by the Lee and Looi score, which was considered perfect for cases with obstructive jaundice and BA patients [21]. Fibrosis should be staged as: 1 – no bridging and fibrosis is limited to portal tract, 2 – porto-portal bridging less than 50%, 3 – porto-portal bridging more than 50%, and 4 – developing cirrhosis.
Ethics approval and consent to participate
Following a succinct and clear explanation of the study’s objectives, the participant’s legal guardian gave signed informed consent. All procedures were carried out in line with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards, as well as the ethical requirements of the institutional and/or national research committee. The study plan was approved by the local ethical scientific committee of the Menoufia Faculty of Medicine (NLI IRB protocol approval number: 00436/2023).
Patients’ inclusion and exclusion criteria
All patients were identified using the inclusion and exclusion criteria. Patients who were simultaneously afflicted with heart disease, acute febrile illness, or skin rash were excluded from the study due to their possible impact on the final AST and platelet results. On the other hand, we excluded any case with post-Kasai (successful or failed) or other causes of neonatal cholestasis other than BA, and also any case of neonatal cholestasis other than BA, for example galactosemia, progressive familial intrahepatic cholestasis, tyrosinemia, inspissated bile syndrome and any neonatal cholestasis caused by sepsis or congenital infection.
Data obtained for all the studied pediatric patients
Clinical (age and sex), family history of similar conditions, laboratory data and ultrasonographic data were obtained from the medical records. Laboratory investigations included complete blood count (CBC) comprising hemoglobin (Hb), white blood cells (WBCs), platelets (PLT), international normalized ratio (INR), total protein by Sysmex Xp 300 (Sysmex Company, Germany), serum bilirubin (total and direct) and serum albumin analyzed by Cobas 111 analyzer, and kidney function tests (serum urea and creatinine) by CX9 Beckman coulter auto analysis. Also, APRI and FIB-4 indices we calculated for all children under study.
Anesthesia protocol
Infants were fasting according to type of food before surgery (2 hours for clear fluids, 4 hours for breast milk and 6 hours for milk formula).
Induction of general anesthesia was by inhalation of 80% oxygen/air with sevoflurane rapid induction technique. The anesthesia depth is clinically adjusted according to monitoring. An intra-venous cannula was inserted and fentanyl (1 µg/kg) and rocuronium (0.9 mg/kg) administered to facilitate tracheal intubation with an appropriately sized tracheal tube for each infant. Maintenance with a mixture of 50% oxygen/air and sevoflurane (1 MAC by gas analysis; end tidal concentration). Pressure regulated volume controlled (PRVC) was the mode chosen for all cases. Standard anesthesia monitors established end tidal CO2 between 25-35 mmHg (GE Datex Ohmeda S/5 Anesthetic 2 Delivery unit system, Arizant, USA). After induction of anesthesia, a 4 F central venous catheter (AMECATH, 10th of Ramadan City, Egypt) single lumen central venous catheter was deployed via the right internal jugular vein by ultrasound.
Fluid maintenance and hemodynamic monitoring
The studied infants were monitored using noninvasive ICON cardiometry (OSYPKA medical). The infant’s data (age, weight, and height) had to be registered. An infusion of Ringer’s acetate solution was administered intraoperatively at a constant rate (6 ml/kg/h) via an infusion pump to cover the fluid deficit and basal fluid requirements.
Surgical procedure
We entered the abdomen through a small right hypochondrial skin incision. The abdomen is grossly inspected for any associated anomalies such as pre-duodenal portal vein or bowel malrotation. Usually, the liver appears cholestatic or fibrotic with absent or atretic gallbladder; but sometimes in type II BA there is a normal gallbladder or hilar cyst. For confirmation of diagnosis of BA, an intraoperative cholangiogram is routinely performed through the gall bladder. Absence of any biliary system can be seen in classic type III BA, while in type II we can see the dye in the gallbladder and common bile duct, but no dye reaches the liver. With confirmation of BA, we start mobilization of the liver for better access of the porta hepatis through the wound. The gallbladder remnant is dissected with the fibrotic parts of the common hepatic duct and common bile duct until we reach the fibrous mass at the porta hepatis between the bifurcation of portal vein, which is cut using a knife or sharp scissors and sent for histopathology. The Kasai portoenterostomy anastomosis is done between the hilum of the liver and 40-50 cm Roux-en-Y retrocolic jejunal loop, hoping for biliary drainage from small ductules in the porta hepatis. In some cases of BA type II, we do cholecysto-porto-enterostomy between the gallbladder and porta hepatis. At the end of surgery a wedge liver biopsy is taken for histopathology.
Calculation of APRI and FIB-4 indices
Complete venous blood samples were taken from every participant, including PLT, and routine blood tests were analyzed. The following liver biochemistry parameters were determined: aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), total cholesterol, AST to platelet ratio index. The APRI and FIB-4 were obtained by the following formulas [9].
Outcomes of the study:
calculation of both APRI and FIB-4 indices in fibrosis among patients with BA,
assessment of the relation between APRI and FIB-4 indices and severity of fibrosis among children with BA,
assessment of the relation between APRI and FIB-4 indices and biopsy findings,
assessment of the relation between APRI and FIB-4 indices and diagnosis of BA.
assessment of the relation between APRI and FIB-4 indices and mortality among children with BA,
validity of APRI and FIB-4 indices in prediction of fibrosis in patients with BA.
Sample size estimation
Sample size was calculated using PASS 11.0 and based on a past review of the literature by Grieve et al. [22], who concluded that APRI is a useful adjunct in evaluating the severity of liver disease in BA at presentation. Sample size was calculated using the following equation: n – (X2 × P × Q)/D2 at CT 95%. Assuming = 0.05 (standard value of 1.96), we calculated that we would need 121 children with BA to achieve a power of 80% (0.8).
Statistical analysis
Data were analyzed using IBM SPSS software package version 20.0 (Armonk, NY, IBM Corp.). The Kolmogorov-Smirnov test was used to verify the normality of the distribution of variables. Comparisons between groups for categorical variables were assessed using the chi-square test (Fisher’s exact test) and continuous variables were compared using Student’s t-test or the Mann-Whitney (t) test. One-way ANOVA (F) used to collectively indicate the presence of any significant difference between several groups for a normally distributed quantitative variable. Receiver operating characteristic (ROC) analysis of APRI and FIB-4 indices was performed for prediction of fibrosis in patients with BA. The significance of the obtained results was judged at the 5% level.
Results
A flowchart of the study population is shown in Figure 1. Of the 142 patients with BA at the National Liver Institute, Menoufia University, 21 patients were excluded from the study (11 patients declined consent and 10 patients did not meet the inclusion criteria, and 121 patients were willing to participate in the study and were divided into five groups: BA type I (n = 19), type II a (n = 2), type II b (n = 45), type III (n = 15) and neglected BA (n = 40) (Fig. 1).
There were no significant differences among BA type I, type II a, type II b, type III and neglected BA regarding age, gender and onset of jaundice and hepatomegaly (p > 0.05), while splenomegaly was the most common among BA type I, type II a, type II b and type III. Also, splenomegaly was the most common among neglected BA, with a significant difference (p < 0.001) (Table 1).
Table 1
Demographic and clinical data among the studied groups (N = 121)
Additionally, no significant differences were found among BA type I, type II a, type II b, type III and neglected BA regarding total bilirubin, direct bilirubin, GGT and ultrasonogram of spleen (p > 0.05), while alkaline phosphatase (ALKP) was significantly higher among neglected BA than BA type I, BA type III, type II b and type II a (p = 0.002). Also, APRI score was significantly higher among neglected BA than BA type II a, BA type III, type II b and type I (p = 0.001). In addition, FIB-4 was significantly higher among neglected BA than BA type II a, BA type II b, type III and type I (p = 0.001), while US of liver was significantly higher among neglected BA than BA type I, BA type II a, type II b and type III (p = 0.002) (Table 2).
Table 2
Laboratory and radiological investigation among the studied groups (N = 121)
There were no significant differences among BA type I, type II a, type II b, type III and neglected BA regarding bile duct proliferation, bile plugs, portal fibrosis, giant cell hepatitis and rosetting (p > 0.05), while degree of fibrosis showed a significant relation among the studied groups (p < 0.001). Also, fibrous expansion of most portal tracts was the most common in BA type I (42.11%), type II a (100%), type II b (40%) and neglected BA (65%). Moreover, focal porto-portal bridging was the most common in type III (66.67%) (Table 3).
Table 3
Biopsy finding among the studied groups (N = 121)
There was no significant difference among the studied groups regarding total bilirubin, direct bilirubin, ALKP, GGT US of liver and US of spleen (p > 0.05) (Table 4). Rosetting was most common in BA type II a (100%), type I (73.68%), type III and type II b (40%) with a significant difference (p = 0.039). Degree of fibrosis was significantly related with the studied groups (p = 0.009). Fibrous expansion of most portal tracts was the most common in BA type II a (100%), type I (42.11%) and type II b (40%). Focal porto-portal bridging was the most common in type III (66.67%), while there were no significant differences among the studied groups regarding bile duct proliferation, bile plugs, portal fibrosis and giant cell hepatitis (p > 0.05) (Table 5).
Table 4
Laboratory and imaging investigations among the studied groups (N = 81)
Table 5
Biopsy finding among the studied groups (N = 81)
The APRI and FIB-4 were significantly related to the diagnosis of BA where APRI score and FIB-4 were higher in type II a than type II b (Table 6). According to the outcome, APRI score was higher in failed than successful (Table 7), while FIB-4 was higher in successful than failed, with no significant difference (Table 8). There was a significant relation for APRI score and FIB-4 according to bile plugs. Where present, bile plugs were associated with a significantly higher APRI score and FIB-4 than in the case of absent bile plugs, while there was no significant relation regarding other biopsy findings (Table 9).
Table 6
APRI score and FIB-4 in relation to the diagnosis of biliary atresia (N = 81)
Table 7
Biopsy finding in relations to outcome (N = 81)
Table 8
APRI score and FIB-4 in relation to outcome (N = 81)
| Parameter | Outcome | U | value | 95% CI | ||
|---|---|---|---|---|---|---|
| Successful (n = 29) | Failed(n = 52) | Lower | Upper | |||
| APRI score | ||||||
| Mean ±SD | 1.22 ±0.70 | 1.46 ±0.99 | 1.224 | 0.225 | –0.15 | 0.61 |
| FIB-4 | ||||||
| Mean ±SD | 12.37 ±19.37 | 9.81 ±5.96 | 0.692 | 0.494 | –10.08 | 4.97 |
Table 9
Biopsy finding in relation to APRI score and FIB-4 (N = 81)
ROC curve analysis showed that the cutoff point of APRI score in prediction of fibrosis in patients with BA was 1.29, with sensitivity of 88.6% and specificity of 76.0% at AUC of 0.650, while the cutoff point of FIB-4 in prediction of fibrosis in patients with BA was 9.82 with sensitivity of 89.0% and specificity of 70.0% at AUC of 0.651 (Table 10, Fig. 2).
Table 10
Validity of APRI Index and FIB-4 Index in prediction of fibrosis in patients with biliary atresia
Fig. 2
ROC analysis of APRI Index and FIB-4 Index for prediction of fibrosis in patients with biliary atresia
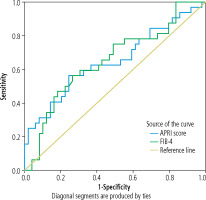
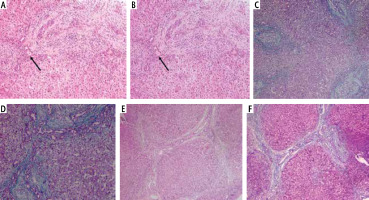
Fig. 3
Liver biopsy. A) A case of biliary atresia (BA) with moderate fibrosis with porto-portal bridging, portal tract showing edema and bile ductular proliferation (H and E 40×; black arrow: intraluminal bile plug). B) A case of BA with moderate fibrosis with porto-portal bridging, portal tract showing edema and bile ductular proliferation (H and E 100×; black arrow: intraluminal bile plug). C) Another case of BA with mild fibrosis limited to portal tract with short septa (Masson Trichrome 40×). D) Another case of BA with moderate fibrosis with porto-portal bridging (Masson Trichrome 100×). E) A case of BA, wedge liver biopsy showing marked fibrosis with incomplete cirrhotic nodule, portal tract showing edema and bile ductular proliferation (H and E 40×), and F) The previous case (Masson Trichrome 40×)
Discussion
Biliary atresia is a severe cholestatic disease of infancy causing neonatal jaundice and is characterized by fibrous obliteration of the biliary tree [23]. Various etiologic mechanisms for BA have been postulated. However, the exact cause of BA remains unknown [24]. Early management of the underlying etiology can postpone and even limit the progression of fibrosis; however, it cannot always prevent continuation to cirrhosis. Currently, liver biopsy is the gold standard for evaluation of the severity of liver fibrosis and cirrhosis [25]. For the proper diagnosis and treatment of chronic hepatic disorders, the application of non-invasive procedures and avoidance of several liver samplings as much as possible are essential [26]. Serological tests such as the APRI and FIB-4 score have been used as less-invasive alternatives to liver biopsy for monitoring liver function in various chronic liver diseases [27, 28]. So, this study aimed to evaluate the validity of the APRI Index and FIB-4 Index in prediction of fibrosis in patients with BA.
The current study showed that APRI score was significantly higher among neglected BA than BA type II a, BA type III, type II b and type I. In this regard, in a study by Kim et al. [29], in which 35 patients with BA were enrolled, clinical outcomes of patients were significantly different between cirrhotic and non-cirrhotic groups based on APRI. Therefore, they considered APRI as a useful tool for assessing liver fibrosis without additional risks in patients with BA during postoperative follow-up care. In a large study in China performed by Yang et al. [30], medical records of 153 infants with BA were reviewed and the efficacy of APRI for diagnosis of liver fibrosis was assessed. In their study, the mean APRI value was 0.76 in the no/mild fibrosis group, 1.29 in the significant fibrosis group (F2-F3), and 2.51 in the cirrhosis group (F4). Also, Imanzadeh et al. [31] reported that higher APRI levels were seen in patients with significant fibrosis and the cirrhotic group. Furthermore, other variables such as gestational age, birth weight, positive C reactive protein (CRP), the need for respiratory support, liver biopsy categorizations, HIDA scans, and cholangiography (based on patency of the bile duct) were all associated with the final diagnosis. Additionally, Chrysanthos et al. [32] found that when using the APRI alone, the stage of fibrosis is incorrectly classified in 40-65% of patients. The diagnostic accuracy of APRI was improved in a study by Lok et al. (33), after incorporation of ALT and the international normalized ratio (INR). Furthermore, the APRI was also found to be of high diagnostic accuracy in assessing the progression of fibrosis in post-liver transplant patients [34].
The present study showed that hepatomegaly was significantly increased among neglected BA than BA type I, BA type II a, type II b and type III. A recent study by Hwang et al. [35] reported that, although their data showed high AUCs for serologic markers in determining liver fibrosis, US elastography is likely to have more clinical benefits than serologic markers because it can provide additional anatomical information, measure hepatic fibrosis, and directly visualize hepatic complications such as ascites, portosystemic collateral vessels, and (rarely) hepatocellular carcinoma.
In the present study, the degree of fibrosis was significantly related to the studied groups. Fibrous expansion of most portal tracts was the most common in BA type II a (100%), type I (42.11%) and type II b (40%). Focal porto-portal bridging was the most common in type III (66.67%). However, there were no significant differences among the studied groups regarding bile duct proliferation. In a study by El-Araby et al. [36] different grades of liver fibrosis were observed in all cases in the early stages of BA (59±12 days). Fibrosis progressed after a short duration (5-31 days, median: 14 days) to significantly higher grades. They also concluded that a high grade of fibrosis is a feature of BA. Interestingly, fibrosis progression was not correlated with the age of the infant or with the interval between the two biopsies. Thus, fibrosis progression in BA is not related to time alone. Unique factors could drive this rapid progression that could even lead to a more advanced grade of fibrosis in a younger infant when compared with that in an older infant. Also, Kim et al. [29] reported that in BA, liver fibrosis may progress rapidly and even cirrhosis may ensue at as early as 2 months of age. Rastogi et al. [37] and Russo et al. [38] also reported high grades of fibrosis in the early stage of BA. Kandil et al. [39] found that more than 50% (53.1%) of BA cases showed marked portal fibrosis compared with 3.4% in the non-BA group, with a highly statistically significant difference between the two groups. This result is similar to that of Rashed et al. [40], who found that 55.2% of BA cases showed bridging fibrosis. Moreover, Rastogi et al. [37] found that portal fibrosis was significantly more frequent in BA compared with other groups. Regarding bile duct proliferation, in a study by El-Araby et al. [36] they observed a significant temporal increase in bile ductular proliferation (BDP), cellular infiltrate, and fibrosis within a short time interval (5-31 days) in the univariate analysis. Fibrosis and BDP increased by more than 60%. However, in the multivariate regression analysis, BDP was the only histopathological finding that showed a significant temporal increase. They also found that BDP was the only independent factor showing a temporal histopathological increase in BA cases within a short time interval, which puts this histopathological feature in the scope of interest in this devastating disease.
The current study showed that APRI score and FIB-4 were significantly related to the diagnosis of BA where APRI score and FIB-4 were higher in type II a than type II b. In agreement with our study, Hwang et al. [35] noted that both the FIB-4 score and APRI positively correlated with progressing clinical categories and showed good to excellent performances for distinguishing between different clinical categories in the present study. Also, Elhenawy et al. [41] stated that APRI and FIB-4 were significantly correlated with fibrosis in BA and were significantly higher in those with advanced fibrosis (Russo F4 and F5), and they stated that these non-invasive serological markers, which are derived from simple routine laboratory tests, may be of help in predicting advanced fibrosis and in long-term follow-up of infants with BA and minimize the need for repeated follow-up liver biopsies. However, previous reports indicate that the FIB-4 score is unreliable for predicting liver fibrosis in BA and other pediatric liver diseases [27, 42, 43]. The APRI has also shown inconsistent efficacy when used for evaluating liver fibrosis in children [27, 42, 43].
In this study ROC curve analysis showed that the cutoff point of the APRI score in prediction of fibrosis in patients with BA was 1.29, with sensitivity of 88.6% and specificity of 76.0% at area under curve (AUC) of 0.650. In a study conducted by Khosravi et al. [44], in King’s College Hospital, UK, a total of 260 patients with BA were evaluated. Their APRI correlated with age, spleen size, and bilirubin. The cutoff value of APRI in this study was about 1.22 and showed a sensitivity of 75% and a specificity of 84% for macroscopic cirrhosis. Many conditions can affect the level of liver enzymes and platelet counts [36]. Additionally, Yang et al. [30] found that the sensitivity, specificity, and accuracy of APRI in diagnosing liver cirrhosis in children with BA when APRI ≥ 1.66 are 70.6%, 82.7%, and 82.4%, respectively. Moreover, in a meta-analysis that included 40 studies by Lin et al. [45], it was found that APRI score > 1.0 had 76% sensitivity and 72% specificity for predicting cirrhosis. Additionally, APRI scores > 0.7 had a 77% sensitivity and 72% specificity for predicting significant hepatic fibrosis.
The current study showed that the cutoff point of FIB-4 in prediction of fibrosis in patients with BA was 9.82 with sensitivity of 89.0% and specificity of 70.0% at AUC of 0.651. In this regard, a study by Behairy et al. [46] found that, regarding the performance of FIB-4, the cutoff point to detect early fibrosis (F1–F2) was > 0.019 with AUC 0.750, and to detect advanced fibrosis (F3–F4), it was > 0.045 with AUC 0.853. These results agree with Elhenawy et al. [41], who reported that the AUC of FIB-4 was 0.0098 with AUC 0.644, 61.9% sensitivity, and 61.9% specificity to discriminating advanced fibrosis. More evidence came from the study by Pokorska-Spiewak et al. [47], where FIB-4 at a cutoff point of 0.18, with AUC 0.708, 85.7% sensitivity, and 93.7% negative predictive value (NPV), helped in detecting any stage of fibrosis; the cutoff point was 0.09 with AUC 0.586. On the other hand, Chen et al. [42] reported that FIB-4 did not correlate with the fibrosis stage. This may be due to the small number of patients in that study (n = 24).
Conclusions
From our results, the cutoff point of APRI score in prediction of fibrosis in patients with BA was 1.29, with sensitivity of 88.6% and specificity of 76.0%, while the cutoff point of FIB-4 was 9.82 with sensitivity of 89.0% and specificity of 70.0% for prediction of fibrosis in patients with BA. Our study confirms that FIB-4 and APRI scores are both able to predict severe fibrosis. APRI score and FIB-4 are good non-invasive alternatives to liver biopsy in the detection of liver fibrosis and its extent in patients with BA.