Introduction
The incidence of renal tumours worldwide is increasing annually. Currently, the annual incidence of malignant renal tumours in Western countries is increasing at a rate of 2% per year [1]. In 2019, approximately 7380 newly diagnosed cases and 14,770 deaths were reported in the United States [2]. Currently, diagnosing renal tumours relies mainly on imaging examinations such as ultrasonography (US), abdominal computed tomography (CT), and abdominal magnetic resonance imaging (MRI). However, imaging examinations have a limited capacity to recognise rare kidney tumours, and a pathological diagnosis cannot be obtained. With advancements in needle biopsy technology, clinicians are increasingly conducting percutaneous renal mass biopsies (PRMBs). This is especially true for specific cases, such as patients with small renal masses, patients who are not candidates for surgery and therefore must undergo minimally invasive treatment, and patients with suspected metastases. In addition, ablative therapies, such as cryoablation, radiofrequency ablation (RFA), and microwave therapy, have been gaining acceptance with growing evidence. PRMB is recommended when patients undergo ablation or active surveillance (AS). For this study, we retrospectively collected clinical information on patients who underwent imaging-guided PRMB at our hospital and evaluated the diagnosis rate, pathology reports, and complications. To provide guidance to clinicians performing PRMBs, the predictors of a diagnostic biopsy were further analysed and a nomogram was constructed. Furthermore, in this study, we analysed changes to the treatment plan based on PRMB results in detail.
Aim
This study aimed to evaluate the predictors of diagnostic imaging-guided PRMB, its accuracy and safety, and subsequent changes to the treatment plan.
Material and methods
Patient selection
For this study, we retrospectively collected the clinical data of patients who underwent PRMB at the Fujian Provincial Hospital between January 2012 and June 2022. The exclusion criteria were as follows: 1) patients with incomplete clinical data and 2) patients who had undergone previous surgical treatments for renal cancer and were found to have tumour recurrence.
Clinical data
The following clinical data were collected for each patient: sex, body mass index (BMI), age, renal tumour diameter, renal tumour side, exophytic appearance (yes/no), number of biopsy cores, tumour features (solid/cystic-solid), and length of biopsy specimen. Pathological information, including malignant vs. benign diagnosis, histologic type, and histologic grade, was also collected. We also collected data on complications, such as perirenal haematoma, sub-renal haematoma, haematuria, backache, pneumothorax, and needle tract seeding, after biopsy based on symptoms and imaging.
PRMB procedure
For PRMB procedures operated by an ultrasonographer (US guide) or a radiologist (CT guide), patients were placed in the lateral decubitus position according to the side of the patient’s renal tumour, and the needle position and direction were guided by US or CT. An 18G lateral cutting through-type autobiopsy gun (BARD Company, New Jersey, USA) was used. After the biopsy, all patients were transferred to the ward for vital sign and coagulation marker monitoring and for a dynamic review of routine blood testing. The patients were required to stay in bed for 1–2 days and received symptomatic treatment for haemostasis and pain as necessary.
Grouping
Patients with biopsy pathology results that distinguished benign vs. malignant tumours were included in the diagnostic group. Those patients with biopsy pathology results that were unable to provide a benign vs. malignant tumour diagnosis were included in the non-diagnostic group.
Additionally, the patients whose treatment plans changed due to the biopsy pathology results were included in the change in treatment group. A change in treatment plan was defined as the addition or deletion of any of the following items in the preoperative treatment plan: surgery, radiotherapy and chemotherapy, targeted therapy, and ablation therapy as well as changes to the surgical plan, targeted therapy plan, or radiotherapy and chemotherapy plan. Patients whose treatment plans were not changed based on the biopsy pathology results from that which was formulated before the PRMB were included in the no change in treatment group.
Statistical analysis
SPSS (version 21.0; IBM Corp, Armonk, NY, USA) was used for all statistical analyses. Continuous variables were reported as mean ± SD and/or median (interquartile range [IQR]) and analysed using the Mann-Whitney U or Kruskal-Wallis test, as applicable. Univariate and multivariate logistic regression models were conducted to determine the independent association between the variables and the diagnostic pathological findings. All statistical tests were 2-tailed, and statistical significance was set at p < 0.05. The concordance rate with the surgical excision diagnosis was also calculated. The R program (version 4.2.1) was used for the nomogram.
Results
Clinical data
A total of 158 patients were included, of whom 115 were male. The median age was 63 years (IQR, 2–86 years). Treatments differed among the patients, including surgery (47 cases), radiofrequency ablation (62 cases), and conservative therapy or others (59 cases). A total of 149 patients underwent biopsy under ultrasound guidance, and 9 patients underwent biopsy under CT guidance (Table I). The diagnostic rate was 85.4% (135/158). There were 135 and 23 patients in the diagnostic and non-diagnostic groups, respectively.
Table I
Baseline patient and lesion characteristics
Biopsy pathology results in the diagnostic group
The pathology results for the diagnostic group are presented in Table II. For 20 cases of renal cell carcinoma, further classification of the histologic type failed. A histologic grade was confirmed in 52 (94.5%) cases. Three cases could not be graded due to insufficient tissue. Forty-seven patients in the diagnostic group underwent surgery and were diagnosed histologically. With postoperative pathology as the gold standard, the concordance between biopsy and postoperative pathology at identifying malignancies, histologic type, and histologic grade was 100% (47/47), 85.1% (40/47), and 54.1% (20/37), respectively.
Table II
Pathology 13 of biopsy in the diagnostic group
Factors associated with a diagnostic PRMB and nomogram
Table III shows the univariate and multivariate analyses of the diagnostic PRMB results. The univariate analysis showed that tumour diameter (p = 0.031) and number of biopsy cores (≥ 2 vs. 1, there was no difference in the diagnostic performance between ≤ 3 and > 3 biopsy cores) (p = 0.001) were significantly associated with a diagnostic biopsy, while greater diameter and larger number of biopsy cores (≥ 2 vs. 1) had p-values < 0.05 in the univariate analysis and were thus also included in the multivariate logistic regression analysis. The multivariate analysis results showed that the greater diameter (odds ratio [OR] = 1.215, 95% confidence interval [CI]: 1.008–1.463, p = 0.041) was an independent predictive factor for a diagnostic biopsy.
Table III
Univariate and multivariate analyses of factors associated with a diagnostic biopsy at the time of the initial renal tumour biopsy
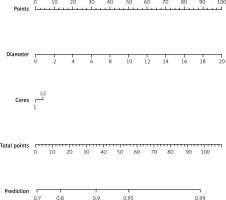
We also used the R package “rms” to produce a nomogram. The nomogram, which included tumour size (diameter) and number of biopsy cores, was constructed to predict a diagnostic biopsy (Figure 1).
Changes to the treatment plan after biopsy
Because the biopsy pathology results were not consistent with previous diagnoses based on imaging results, the treatment plans of 15 (9.5%) patients changed. Further details are provided in Table IV.
Table IV
Changes to the treatment plan based on PRMB results
PRMB complications
Complications occurred in 14 (8.9%) patients. Among them, 6 patients with perirenal haematoma and 2 with subcapsular haematoma recovered without additional treatment. Post-procedure transient fever occurred in 1 patient, and gross haematuria occurred in 1 patient; however, the symptoms disappeared spontaneously without treatment for both these patients. One patient developed symptoms indicative of urinary tract infection (frequent urination and urgency) after the procedure, which was managed with oral antibiotics. Two patients experienced lumbago after the procedure, both of whom recovered after analgesic treatment (Table V).
Table V
Adverse events reported after renal tumour biopsy
The severity of complications was based on the Clavien-Dindo grading system. Fourteen cases were Clavien-Dindo grade 1, and 2 cases were Clavien-Dindo grade 2. There were no serious complications (Clavien-Dindo grade > 2), and no needle tract seeding was found after a median follow-up time of 37 months (IQR, 12–56 months).
Discussion
The detection rate of renal tumours has increased recently. Previously, the majority of renal tumours were believed to be malignant, and treatment was thus relatively radical. However, as the number of renal tumours detected have increased, many studies have found that benign tumours are not rare (20–30%) [3–5]. Although most malignant renal tumours can be identified by advanced imaging (e.g. CT, MRI), some renal malignancies cannot be differentiated because secondary changes, such as internal haemorrhage, necrosis, and cystic changes, occur less frequently. Additionally, some benign renal tumours, such as fat-deficient renal hamartoma, metanephric adenoma, and eosinophilic adenoma, have findings on imaging that are similar to those of renal cell carcinoma. Bauman et al. reported that approximately 14.1% (129/916 cases) of benign renal tumours confirmed by postoperative pathology are misdiagnosed as malignant on imaging [6]. PRMB can not only compensate for these deficiencies in imaging techniques, but also help clinicians obtain pathological information in advance to guide treatment, and thus it is gradually being accepted and used clinically.
With the advancement of biopsy equipment and technology, current studies have revealed that the first-time biopsy diagnosis rate is approximately 84.6–92.3% [4, 7–13]. In this study, the first-time biopsy diagnosis rate was 85.4%. In the non-diagnostic group, normal tissue may have been biopsied, or the amount of tissue may have been insufficient for pathological diagnosis. For renal tumours that cannot be diagnosed by first-time biopsy, a second biopsy can be performed, and the diagnosis rate of the second-time biopsy is approximately 80–100% [4, 7, 12, 14]. Currently, a few studies have analysed the predictors of a diagnostic biopsy and have reached different conclusions. A study by Jeon et al. on 393 diagnostic biopsies and 49 non-diagnostic biopsies found that small tumour size, cystic nature, and biopsy during the initial years of the study were independent predictors of a non-diagnostic biopsy [7]. Richard et al. concluded that larger tumour size and an exophytic location were factors associated with a diagnostic biopsy [4].
Posielski’s research suggests that cystic features, hypo-enhancement on imaging, a smaller mass diameter, and a longer skin-to-tumour distance increase the risk of non-diagnostic findings [15]. In this study, the univariate analysis showed that higher tumour diameter and number of biopsy cores ≥ 2 (there was no difference in the diagnostic performance between ≤ 3 and > 3 biopsy cores) were significantly associated with a diagnostic biopsy, and the multivariate analysis showed that greater tumour diameter was an independent factor associated with a diagnostic biopsy. Furthermore, we produced nomograms to predict the diagnostic biopsy for the first time.
As an invasive method of diagnosis, PRMB can provide important information for clinical decision-making, such as distinguishing a benign vs. malignant lesion, histopathologic type, and renal cancer grade. Current studies show that the concordance between biopsy and postoperative pathology at differentiating benign from malignant renal masses is approximately 97.1–100% [7, 8, 12, 14, 16], and the concordance between biopsy and postoperative pathology of diagnosing the renal tumour type is approximately 91–98% [4, 7, 14, 16, 17], both of which are quite high. However, the concordance between biopsy and postoperative pathology of grade diagnosis is unsatisfactory, at approximately 46–69% [4, 7, 12, 14, 18]. A meta-analysis by Marconi et al. found that the sensitivity and specificity of diagnostic PRMB were 99.1% and 99.7%, respectively; in addition, good (k = 0.683) and fair (k = 0.34) agreement were observed between histologic subtype and Fuhrman grade on PRMB and surgical specimens, respectively [13]. In this study, 47 patients in the diagnosis group underwent surgery, and, with postoperative pathology as the gold standard, the concordance between biopsy and postoperative pathology at differentiating benign and malignant lesions, typing, and grading were 100%, 85.1%, and 54.1%, respectively.
In recent years, in addition to traditional surgical treatments (e.g. open and laparoscopic radical nephrectomy, partial nephrectomy), various minimally invasive treatment options have gradually been adopted for early renal tumours, such as cryoablation, RFA, and microwave ablation. AS for renal masses has become an acceptable initial management option, especially for patients with small tumours (< 2 cm) and elderly patients (> 75 years old) or those with various comorbidities [19]. AS is an appealing treatment option for elderly patients with small renal masses (SRMs) because it eliminates the risks associated with primary interventions without compromising survival outcomes [20, 21]. For some renal tumours with smaller diameters, it is relatively difficult to use imaging alone to distinguish benign from malignant cases. However, if pathological information can be obtained through PRMB, the treatment plan can be altered accordingly. In this study, 15 (9.5%) patients had their treatment plans changed based on the biopsy pathology results, which were different from the imaging diagnosis results (Table IV). Some recent studies suggest that PRMB results can lead to some changes in the treatment plan. In a study conducted by Richard et al., the authors assumed that a benign renal tumour diagnosis on biopsy always resulted in treatment plan changes; thus, the proportion of patients with changes in their treatment plans was reported at 26% [4]. Azawi et al. reported that treatment plans changed in 35% of patients with benign findings or those with non-renal cell cancers on biopsy [22]. We do not agree with these previous studies. Rather, we believe that not all benign tumours are exempt from surgery, and each case with changes to the treatment plan should be analysed separately, as was done in the current study (Table IV). For renal tumours, not only can pathology results be obtained through PRMB, but also the punctured tissue can be used for genetic examinations so that the pathological results and biological characteristics can be used to formulate personalised and specific treatment plans.
Common complications of PRMB include renal haemorrhage, peripheral renal haemorrhage, postoperative pain, haematuria, pneumothorax, needle tract seeding, and puncture site infection. With advancements in imaging technology and the application of automatic biopsy guns, the incidence of these complications has significantly reduced. Bleeding in the kidney and surrounding tissue accounts for 75% of all complications, with an incidence of 2.1–7.6%, most of which can be managed with conservative treatment, while a few may require interventional therapy. However, other serious complications such as persistent haematuria, massive retroperitoneal bleeding, and arteriovenous fistulas are rare [8, 15, 23, 24]. In this study, 8.9% patients developed postoperative complications, mainly perirenal haematomas (42.9%). Serious complications (Clavien–Dindo classification > grade 2) in the postoperative period were not observed, and no needle tract seeding was observed in the follow-up. It is worth noting that needle tract seeding is a serious complication, and although the incidence is extremely low, cases are reported periodically [25–27]. Based on our research results and relevant recent literature, PRMB is a relatively safe method of diagnosing renal tumours.
This study had several limitations. First, some patients underwent ultrasound-guided RFA after PRMB rather than surgery, resulting in a few patients with postoperative pathology. Therefore, our conclusions regarding the diagnostic accuracy of typing and grading should be interpreted with caution. However, our findings are consistent with those of previous studies [4, 7, 14, 18]. This single-centre retrospective study was less convincing than a prospective randomised controlled trial and the surgeon’s experience may also limit the generalisability of findings.
Conclusions
Our study indicated that imaging-guided PRMB is safe and has high diagnostic accuracy, and the tumour diameter is independent predictor of a diagnostic biopsy. PRMB could be used as a supplement to imaging techniques, and it may be particularly useful for inconspicuous and rare types of lesions. Although our results are satisfactory, more data are needed to draw more conclusions.
Ethics approval and consent to participate
The authors indicate this study was in accordance with the ethical standards of the World Medical Association (Declaration of Helsinki), and the study received permission from the Ethics Committee of Fujian Provincial Hospital (K2021-08-010). This study was a retrospective study. The data are anonymous, and the requirement for informed consent was therefore waived.