A 79-year-old man was admitted to our hospital on July 27th, 2023, with sudden chest pain. The electrocardiogram revealed a new-onset left bundle branch block. The echocardiographic examination evidenced mildly reduced ejection fraction (EF). The patient’s past medical history included multiple percutaneous coronary interventions (PCI): in 2007, a PCI of the left anterior descending artery was performed, with the discovery of a chronic total occlusion of the right coronary artery (RCA). In 2021, the patient underwent a PCI of the circumflex artery and an unsuccessful attempt to revascularize the RCA. In 2022, a successful PCI of the RCA was completed.
The angiographic examination performed at the admission revealed subocclusive stenosis at the proximal and distal edges of the previously implanted RCA stent. In addition, haziness was observed in the mid segment of the RCA (Figure 1 A), suggesting a possible complication. This led to the need for a differential diagnosis between coronary perforation and coronary pseudoaneurysm (PSA), both of which are known complications of stent thrombosis (ST). Echocardiography excluded the presence of pericardial effusion.
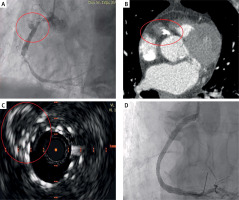
Figure 1
A – Proximal and distal stent thrombosis of right coronary artery associated with haziness. B – Angio-computed tomography confirming the pseudoaneurysm and the proximal leak. C – Complete exclusion of the pseudoaneurysm at intravascular ultrasound and good apposition of the implanted stents. D – Final angiographic result
We performed balloon dilation of the subocclusions and deployed a 3 × 15 mm zotarolimus-eluting stent at the distal edge of the lesion. Additionally, we implanted a 3.5 × 26 mm cobalt-chromium (CoCr) polytetrafluoroethylene-covered stent to seal the suspected pseudoaneurysm. However, post-procedural angiography revealed persistent inflow into the PSA, prompting the implantation of a second covered stent, sized 4 × 18 mm, to fully seal the defect.
The following day, a contrast-enhanced angio-CT confirmed the diagnosis of PSA and identified a remaining endoleak at the proximal edge of the stent (Figure 1 B). Consequently, we placed another flexible CoCr stent (3.5 × 23 mm) to close the leak. Intravascular ultrasound (IVUS), performed at the end of the procedure, confirmed the complete closure of the pseudoaneurysmal chamber (Figure 1 C). The final angiographic assessment showed an optimal result (Figure 1 D).
PSA formation could be the result of intrinsic vascular fragility combined with procedural factors such as stent malapposition, edge dissection, or even spontaneous dissection that might not be visible on angiography [1]. In our case, the use of IVUS was delayed until the end due to the patient’s initial instability, prioritizing the need to treat the occlusions first and stabilize the vessel.
If left untreated, PSA may rupture, causing cardiac tamponade, or become infected. Furthermore, the turbulent flow within the aneurysmal sac increases the risk of intrastent thrombosis or distal embolization [2]. Unlike coronary aneurysms, which may regress, PSAs often do not resolve and could have a more malignant course [3].
There is no well-established standard for treating coronary pseudoaneurysms due to their rarity. Conservative pharmacological treatment can be considered for asymptomatic patients without signs of angina or myocardial ischemia [4]. However, in symptomatic cases like this one, treatment options include covered or drug-eluting stents, coil embolization, or surgical intervention such as coronary artery bypass grafting with resection or ligation of the PSA [2]. Given the acute scenario, we opted to deploy overlapping covered stents to close the pseudoaneurysm immediately.
Diagnostic approaches for PSA include angiography and intravascular imaging modalities. In this case, IVUS was utilized after angioplasty to confirm the complete closure of the pseudoaneurysmal cavity. The patient was discharged after 17 days of hospitalization with preserved left ventricular ejection fraction (52%). Due to the onset of atrial fibrillation, triple antithrombotic therapy with warfarin, acetylsalicylic acid and clopidogrel was continued for 3 months, followed by a transition to a direct oral anticoagulant and ticagrelor.
At the 6-month follow-up, the patient remained asymptomatic with no evidence of recurrent angina, and echocardiography showed good recovery of left ventricular function.








