Introduction
Acute post-streptococcal glomerulonephritis (APSGN) is a renal immunological complication of an infection caused by nephritogenic strains of group A β-hemolytic streptococci (GAS, Streptococcus pyogenes). Approximately 500,000 new cases of APSGN are diagnosed each year, with over 90% in low-socioeconomic-status regions, with an incidence reaching 28.5/100,000 of the pediatric population/year [1-3]. In the developed countries, a systematic decrease in disease incidence is observed with an estimated incidence (based on Italian data) of 0.3/100,000 of the pediatric population/year [4]. The disease occurs twice as often in boys, most frequently in children between 5 and 12 years of age and after 60 years [2].
Symptoms appear after a latency period after streptococcal infection of 1-3 weeks (streptococcal tonsillitis, scarlet fever) or 3-6 weeks (erysipelas or impetigo) [2, 3]. Clinically, the disease manifests as a nephritic syndrome of varying severity (microscopic or gross hematuria, usually non-nephrotic proteinuria, acute kidney injury [AKI], edema, arterial hypertension), although it may also present as incidentally diagnosed asymptomatic hematuria [3, 5]. The diagnosis is based on the history, typical clinical course, and characteristic pattern of abnormalities in blood tests: elevated antistreptolysin O (ASO) and decreased C3 component of the complement system. Treatment is symptomatic (salt-restricted diet, fluid restriction, hypotensive treatment, rarely renal replacement therapy) [3]. The prognosis in APSGN is usually excellent, with no distant renal sequelae of the disease in most patients [2].
The exact immunological mechanism of the disease is not fully explained yet, and in situ formation of immune complexes containing streptococcal antigen is the most likely pathogenesis. The complexes activate the alternative complement pathway and lead to local glomerular injury. This manuscript aims to present a very severe case of APSGN and summarize current knowledge on the disease, emphasizing immunological mechanisms.
Case report
We present a case of a girl aged five years and ten months admitted to our department with AKI. She presented with weakness, apathy, fever, and vomiting for four days. She did not want to eat or drink, and due to fever, she received acetaminophen (suppositories) 15 mg/kg/dose once daily. On the day of admission, she passed only a small amount of urine in the morning. In addition, she had a history of vulvitis a few days before hospital admission and a history of symptomatically treated respiratory tract infection with a sore throat two weeks before.
The pregnancy was complicated by hyperthyroidism, glucose intolerance, premature uterine contractions, maternal infection in the 34th week of gestation, and fetal distress. The girl was born in the 35th week of pregnancy by cesarean section, and congenital pneumonia, sepsis, intracerebral hemorrhage I/II, and hypothyroidism complicated the perinatal period. The girl remained under endocrinological care and received L-thyroxin in the dose of 12.5 µg daily. Except for varicella eight months before, she did not have any other serious diseases after the neonatal period. She had normal development and received vaccinations according to the Polish schedule. The family history was unremarkable.
The tests taken in an emergency department revea- led leukocytosis 21 × 103/µl, C-reactive protein (CRP) 11.5 mg/dl (normal value < 0.5), procalcitonin 1.93 ng/ml, urea 180 mg/dl, creatinine 4.3 mg/dl (glomerular filtration rate [GFR] according to Schwartz formula 11.2 ml/min/1.73 m2 [6]), ultrasonography showed kidneys of normal size (right kidney 89 mm, left kidney 93 mm), with normal corticomedullary differentiation and increased echogenicity. The girl received 500 ml of intravenous (iv) fluid (5% glucose + saline), one dose of ceftriaxone (70 mg/kg/dose) and was admitted to our nephrology department.
On admission, the girl was in average general condition, apathetic. Physical examination revealed foot and ankle edema, sore throat, symmetrical, swollen tonsils without exudate, normal chest examination, bloated abdomen, and vulvitis. Her height was 117 cm, her weight was 21.4 kg (pre-disease weight approx. 20 kg), blood pressure 115/71 mm Hg, heart rate 80/min, respiratory rate 20/min, temperature 37.3°C. Her laboratory tests in the department are shown in Table 1. The heart and lung X-ray images were normal. The girl received a fluid bolus (250 ml), glucose with insulin, furosemide 50 mg iv. The first urine sample was passed 6 hours after the admission. The urine was cloudy, with proteinuria 543 mg/dl, leukocytes, and erythrocytes completely covering the visual field. The immunological tests were as follows: elevated ASO titer, decreased C3, normal C4 and immunoglobulin levels [IgA – 134 mg/dl (n: 28-235), IgG – 938 mg/dl (n: 853-1440), IgM – 93.1 (n: 36-198)]. The girl was diagnosed with APSGN. During the first days, she required intravenous furosemide to maintain fluid balance. A low-salt and low-potassium diet were implemented. Ion-exchange resins were administered, and ceftriaxone was continued due to very high inflammatory markers. Because of severe hyperuricemia, she received a single dose of rasburicase (0.2 mg/kg/dose iv), which resulted in a significant drop in serum uric acid concentration. The child’s condition improved, and overhydration disappeared in the following days. In laboratory tests, renal function parameters gradually decreased, inflammatory markers and ions normalized, and slight anemia persisted. In urine examination, decreasing proteinuria was observed. Arterial blood pressure periodically rose to 125/75 mm Hg but normalized completely during the hospitalization. On the 12th day in the evening, severe colicky pain in the mediastinum occurred. The ultrasound examination showed a deposit (22 × 11 mm) in the gallbladder. The deposit was most likely related to the administration of ceftriaxone. A pediatric gastroenterologist consulted the child and recommended further observation. On the day of the discharge, she presented in good condition, without edema (body weight 19.7 kg), in morphology there was persistent anemia, her kidney function improved significantly, and her ions normalized; her transaminases and bilirubin were normal, and her γ-glutamyl transaminase was slightly elevated [23 U/l (n: 8-18)]. She was discharged home with persistent proteinuria.
Table 1
Laboratory results in the patient with acute post-streptococcal glomerulonephritis
In further follow-up, her kidney function normalized six weeks after the disease onset (creatinine – 0.4 mg/dl, glomerular filtration rate [GFR] 120.8 ml/min/1.73 m2), urinalysis normalized within six months, and gallbladder deposits disappeared after three months. She had no recurrences of the disease.
Discussion
We reported the clinical course of a very severe case of APSGN. The girl presented with unusually high parameters of kidney function. She did not require renal replacement therapy despite an initial GFR below 15 ml/min/1.73 m2 as her kidney function improved spontaneously. AKI with severe overhydration, hypertension or hyperkalemia indicates acute dialysis; however, this is extremely rare in children with APSGN. Interestingly, the girl presented with high inflammatory markers, suggestive of generalized infection, which does not fit the picture of post-infectious glomerulonephritis. Most likely, her AKI was of mixed etiology: APSGN and superimposed sepsis.
APSGN can occur in sporadic and epidemic forms and is more common in spring or autumn, associated with a higher incidence of streptococcal infections [3]. GAS is classified as different serotypes depending on the M protein present in the cell membrane [7]. Seegal and Earle postulated already in the early 1940s that only certain types of streptococci can cause glomerulonephritis. The types responsible for glomerulonephritis are called “nephritogenic”. The risk of APSGN in case of infection caused by nephritogenic GAS reaches 15% [3]. Different types of S. pyogenes cause APSGN followed by pharyngitis, tonsillitis or scarlet fever (types 1, 2, 3, 4, 6, 12, 18, 19, 24, 25, 31, 49) and other types followed skin infection such as roseola or impetigo (types 49, 55, 56, 57, 60) [2, 3, 5, 8]. It is unclear what determines the potential of only certain streptococcal serotypes to cause APSGN. Streptococcal pharyngitis precedes APSGN more often than dermatitis [1], and serotypes 1, 4 and 12 are responsible for 90% of all APSGN cases [2, 3, 8]. It is worth noting that epidemic cases of APSGN are usually reported after cutaneous infections [2, 9]. It should be emphasized that many cases of the disease are barely symptomatic or even subclinical – in epidemic cases, this may concern as many as 80% of all cases [10, 11]. Therefore, in our opinion, it is reasonable to routinely order a urinalysis after a streptococcal infection taking into account the latency period.
Literature data indicate a twofold higher incidence of APSGN in boys [11]. Although the disease can occur at any age, including in adults (about 10% of cases), it is rarely found in children younger than three years of age, with only isolated reports of patients in the 1st year of life [12]. The smaller pore size in the glomerular basement membrane (GBM) in children, which hinders the renal removal of immune complexes, may be responsible for the higher incidence of APSGN in the pediatric population. On the other hand, the low incidence in the first two years of life may result from a lower frequency of streptococcal infections, the immune system’s immaturity, and lower ability to form immune complexes in this group of patients [2, 13].
Macroscopic hematuria, edema, and arterial hypertension are the most prevalent clinical signs of APSGN, observed in approximately 30-50%, 70%, and 50-90% of the patients, respectively [2, 3, 11]. Nephrotic-range proteinuria is uncommon, observed in approximately 2-5% of cases [3, 14]. Both edema and hypertension result from renal sodium and water retention. In the literature, there are rare cases of acute circulatory failure with pulmonary edema.
Interestingly, reversible systolic or diastolic cardiac dysfunction is present in up to 20% of children with APSGN [15]. Occasionally, there may be a sudden increase in blood pressure with encephalopathy manifesting as severe headaches, vomiting, visual disturbances, and seizures [16]. Our patient presented with severe overhydration (almost 10% of normal body weight) but without evident circulatory insufficiency or pulmonary congestion features. She did not have sustained hypertension either.
Though the disease has been known for decades, the exact immunological mechanism is still unclear. APSGN belongs to the group of post-infectious glomerulopathies (PIGN). It is by far the most common form of this disease, although the condition may be a consequence of other bacterial, viral, or even parasitic infections (Table 2). Non-streptococcal PIGN is found nowadays more commonly in adults, in developed countries, and generally has worse outcomes [14]. Most pathophysiological data are available for APSGN. There are five main mechanisms proposed to explain the immunologic glomerular injury induced by GAS infection: deposition of circulating immune complexes containing streptococcal antigens, in situ immune complex formation resulting from deposition of streptococcal antigens within the GBM, and subsequent antibody binding, in situ glomerular immune complex formation promoted by antibodies to streptococcal antigens that cross-react with glomerular components (molecular mimicry), activation of the alternative pathway of complement due to the transient presence of autoantibodies against factor B, and finally autoimmunological reaction [3, 14, 17, 18].
Table 2
Microorganisms that may cause acute post-infectious glomerulonephritis according to [2, 3, 14, 41-43]
There is some experimental and clinical evidence supporting the autoimmune reactivity theory. Firstly, anti- immunoglobulin G (IgG) reactivity has been revealed in subjects with APSGN. For example, anti-IgG glomerular deposits were detected in renal biopsy specimens, and anti-IgG activity was found in eluates from the kidney of a patient with a fatal course of APSGN [3]. Burova et al. reported that nephritogenic strains of Streptococcus produce IgG Fc binding proteins (IgGFcBPs) and may stimulate the production of anti-IgG antibodies [19]. In addition, streptococcal neuraminidase was found to modify human immunoglobulins (reveal or create hidden antigens or epitopes), which triggers the production of antibodies directed against them. Rodriguez-Iturbe found neuraminidase activity and free neuraminic acid in the serum of patients with APSGN, and this finding was specific for this entity [20]. Also, some patients may have different auto-antibodies such as antinuclear antibodies (ANA), anti-dsDNA, anti-C1q antibodies, antineutrophil cytoplasmic antibodies (ANCA), and rheumatic factor (RF), which suggests non-specific autoimmune reactivity [21]. The pathogenetic role of these autoantibodies is unclear.
The most plausible theory, confirmed by numerous experimental animal studies, is that the immune complexes that form in situ in glomeruli cause APSGN [3, 22]. These complexes contain a streptococcal antigen and immunoglobulins G directed against them. Nephritis-associated plasmin receptor (NAPlr) and streptococcal pyrogenic exotoxin B (SPE B) are the most commonly proposed “nephritogenic antigens”. Also, other streptococcal proteins were suggested as possible triggering antigens, i.e., endostreptosin (ESS), preabsorptive antigen, and nephritogenic-strains associated protein [2, 3, 14]. The isolation of SPE B and NAPlr and antibodies directed against them simultaneously from serum and renal biopsies of patients with APSGN makes them the most serious candidates for the nephritogenic toxin.
NAPlr is a glycolytic enzyme with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and plasmin-like activities [23, 24]. The latter may play a vital role in a glomerular inflammatory reaction, as elevated urinary plasmin activity was found in patients with APSGN [25]. Japanese authors revealed that in kidney biopsy specimens from patients with APSGN, NAPlr was observed mainly in the early stage of the disease (the first two weeks) but was not colocalized with either C3 or IgG. In addition, the authors analyzed the presence of anti-NAPlr antibodies in sera of patients with APSGN, in patients with GAS infection but without glomerulonephritis, and in healthy volunteers. Anti-NAPlr antibodies were present most frequently in subjects with APSGN, and antibody titers were significantly higher than in patients with streptococcal infection or in the control group. Moreover, antibody titers remained elevated during the entire 10-year follow-up period [23].
SPE B has a cationic cysteine proteinase and was found in subepithelial deposits (humps) of biopsied patients with APSGN [26]. Batsford et al. found SPE B in 12 out of 17 kidney biopsy specimen colocalized with complement deposition within the humps. In this study, antibodies to SPE B were detected in sera of all 53 patients who were tested. In contrast, in this patient cohort, antibodies to NAPlr were found in only 5 of 47 tested sera and only one biopsy sample [27]. Interestingly, Batsford’s study included only patients from Latin America and Switzerland. An interesting theory is that various antigens trigger APSGN in different parts of the world or patients with diverse genetic backgrounds [3, 18]. It is also possible that both these antigens play a role in the pathogenesis of APSGN – NAPlr maintains plasmin activity and other streptococcal enzymes (streptokinase, enolase) induce damage to the GBM. Consequently, immune complexes containing SPE B, complement factors, and plasma protein pass through the damaged membrane to form subepithelial humps.
Both SPE B and NAPlr can activate the complement system through an alternative pathway (as evidenced by lowered C3 and normal C4 component levels) and lead to the development of local inflammation by starting chemotaxis and interleukin-6 production by mesangial cells and expression of adhesion molecules on the endothelium [23, 27, 28]. A decrease in complement component C3 is found in 78-96% of patients [2, 3]. SPE B is also a superantigen capable of activating T-cells without antigen-presenting cells [14]. A decrease in C4 has also been described in the literature. It is explained by activating the classical pathway of complement in some patients by circulating immune complexes containing streptococcal antigens [29]. In APSGN, complement may also be activated through the lectin pathway [14]. An increase in IgA and IgG immunoglobulin levels was also found in some patients with APSGN [2, 30]. A summary of the proposed immunological mechanism of APSGN is presented in Figure 1.
Fig. 1
The proposed pathogenesis of acute post-strepto- coccal glomerulonephritis according to [14, 23, 24]
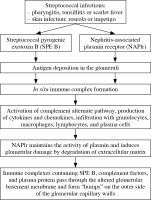
Recently, a novel mechanism of complement activation was proposed. Chauvet et al. found autoantibodies against factor B (a component of the alternative pathway C3 convertase) in 31 out of 34 children with APSGN. Anti- factor B autoantibodies were transient and correlated with plasma C3 and soluble C5b-9 levels. The authors also demonstrated that anti-factor B antibodies enhance alternative pathway convertase activity in vitro, confirming their pathogenic effect. Interestingly, anti-factor B antibodies were absent in children with C3 glomerulopathy – another disease with activation of the alternative complement pathway. In the authors’ opinion, at the onset of a nephritic syndrome with a low C3 level, screening for anti-factor B antibodies might help guide indications for kidney biopsy to avoid misdiagnosed C3 glomerulopathy [31].
The genetic backgrounds that predispose to APSGN are still unclear, but they may explain a greater susceptibility to the disease in some families. Studies focused mainly on human leukocyte antigen (HLA) class II genes, which are crucial in the antigen presentation process. Bakr et al. found that Egyptian children with the allele HLADRB1*03011 or HLA-DRB1*1105 had an increased risk of developing APSGN [10]. On the other hand, Mori et al. found no correlation with any HLA-DRB1 allele, but a significant risk of APSGN was found in Japanese children with HLA-DP5 (HLA-DPA1*02022 and HLADPB1*0501) [32].
Renal biopsy is not a standard diagnostic procedure and is only performed if another glomerulopathy is suspected. In light microscopy, the lesions are generalized and diffuse. Enlargement of glomeruli with the proliferation of endothelial and mesangial cells and numerous inflammatory cells (mainly neutrophils, but also macrophages, lymphocytes, and plasma cells) infiltrating the glomerular vessels and mesangium are characteristic features of APSGN. The accumulation of inflammatory cells in the vascular bundle and the fibrin deposits causes glomeruli enlargement and filling of Bowman’s space. The vascular endothelial cells are swollen, and the capillary lumen narrows. As the patient heals, the number of granulocytes decreases, and the lumen of the capillaries become more visible. The proliferation of mesangial cells lasts the longest. Immunofluorescence examination reveals irregular granular deposits of complement components and antibodies located along the walls of glomerular capillaries, less frequently in the mesangium. IgG and C3 deposits are most commonly seen. Less commonly, C4, C1q, IgM, fibrinogen, and factor B are detected. The scattered distribution of C3 deposition in the capillary wall gives a “starry sky” pattern. In electron microscopy, electron-dense dome-shaped subepithelial granular deposits (humps) are the most characteristic feature. IgG class antibodies, C3 component, and streptococcal toxin SPE B were detected in the humps. Humps, although characteristic for APSGN, are not pathognomonic for this disease because they are sometimes observed in other entities with glomerular deposition of immune complexes, e.g., lupus nephropathy. Electron microscopy may also reveal granular deposits within the mesangium and subendothelial space [2, 3, 14, 17].
The diagnosis of streptococcal etiology is confirmed by the elevated titer of antistreptolysin O (ASO), which is found in virtually all children after a throat infection and 30-50% after a skin infection [7]. In children with skin infections, the determination of anti-deoxyribonuclease B (anti-DNase B), anti-hyaluronidase (AHase), or anti-nicotinamide adenine dinucleotidase antibodies (anti-NADase) is more practical [2, 3]. The titer of the serological markers increases from the seventh day after the infection, reaching a peak around day 30, but returns to the normal range after about 3-4 months [33]. Serological tests for a history of streptococcal infection should be performed as they have a higher sensitivity than a history of a recent infection or positive throat, nose, or skin cultures [2, 17]. Table 3 presents biochemical and immunological abnormalities typical for APSGN with a literature-based duration of normalization. The differential diagnosis of the causes of the nephritic syndrome included (Table 4), e.g., membranoproliferative glomerulonephritis (including a recently separated disease entity – C3 nephropathy), rapidly progressive glomerulonephritis, lupus nephropathy, and other forms of post-infectious glomerulonephritis (PIGN). In the patient described here, immunological testing and the self-limiting course of the disease allowed for a non-invasive diagnosis of APSGN. The most likely, symptomatically treated upper respiratory tract infection was of streptococcal etiology.
Table 3
Biochemical and immunological anomalies found in patients with acute post-streptococcal glomerulonephritis [3, 14, 17]
Table 4
Differential diagnosis of nephritic syndrome (classifications based on typical course)
Treatment of APSGN is purely symptomatic and involves monitoring fluid balance, body weight, blood pressure, and serum creatinine and potassium levels [2, 3, 17]. The dietary sodium intake should be limited, and in the case of a tendency to hyperkalemia also potassium should be limited. In overhydration, fluid restriction and loop diuretics (most commonly furosemide given at an initial dose of 1 mg/kg) are used. In the treatment of hypertension, apart from loop diuretics, calcium channel antagonists may be used; drugs inhibiting the renin-angiotensin-aldosterone system ought to be used with caution due to the tendency to hyperkalemia and risk of exacerbating renal impairment [17]. In the present patient, hyperuricemia was successfully treated with rasburicase. Rasburicase, intended initially for the treatment of tumor lysis syndrome, was found to be effective and safe in children with AKI of different etiology, including hemolytic-uremic syndrome (HUS) [34, 35]. The authors’ center is considering the use of rasburicase in any case of AKI with severe hyperuricemia (usually > 10 mg/dl), and experience to date (mainly in patients with HUS) has been very promising.
Antibiotics are not routinely used in the treatment of APSGN. However, in the case of symptoms of active infection, an antibiotic covering the spectrum of GAS is administered – according to Polish recommendations, it should be phenoxymethylpenicillin administered for ten days at a dose of 100,000–200,000 IU/kg/day [36]. Some authors recommend antibacterial treatment even in the absence of features of active infection in order, among others, to reduce the risk of infection in household members [3]. The patient was started on ceftriaxone due to fever and very high inflammatory markers suggestive of sepsis. We did not detect a particular source or etiologic factor of the present infection, though physical examination revealed vulvitis and pharyngitis. We chose ceftriaxone following Surviving Sepsis Campaign international guidelines [37]. The patient presented with biliary deposits during the treatment. A recently published systematic review including 5717 pediatric patients who received ceftriaxone reported 1136 adverse drug reactions (ADRs). The most frequently reported ADRs were gastrointestinal disorders (37.4%, 292/780), followed by hepatobiliary disorders (24.6%, 192/780). Almost all cases of biliary pseudolithiasis were reversible; however, the incidence was surprisingly high, affecting one in five pediatric patients (20.7%) [38]. Notably, the incidence of biliary pseudolithiasis might be even higher in patients with renal function impairment as drug biliary excretion rises in case of kidney insufficiency.
Although improved hygiene conditions and a decrease in the frequency of skin infections have reduced the incidence of APSGN in developed countries, there is still no clear evidence of a reduced risk of the disease after antibiotic treatment of streptococcal infections [2, 9]. In one Australian observational study, treatment of streptococcal infection with benzathine penicillin G was found to prevent new cases of glomerulonephritis [39]. Experimental data suggest that the widespread presence of fluoride in water decreases virulence factors of GAS and may also contribute to reduced APSGN prevalence in developed countries [40].
The prognosis in children with APSGN is generally excellent. The disease is self-limiting, and in most cases, renal function recovers entirely after the acute period. Long-term outcome studies in children with APSGN are scarce, but the overall prognosis is excellent. Persistent proteinuria, microalbuminuria, or hypertension is found in only 0.5-3% of patients. Isolated hematuria may persist the longest, sometimes up to two or even five years [2, 3, 17]. The risk for renal function impairment is very low, in most studies below 1% [3]. Authors from Great Ormond Street Hospital recommend yearly follow-up after complete recovery to look for evidence of hypertension, proteinuria, or renal impairment [14].


