Vedolizumab (VDZ) is a humanized anti-α4β7 integrin monoclonal antibody approved for treatment of moderate to severe active ulcerative colitis (UC) or Crohn’s disease (CD). It is administered as a 300 mg intravenous infusion, with an induction dose at 0, 2, and 6 weeks and for maintenance, every 8 weeks thereafter [1, 2]. The efficacy and safety of VDZ in adults with IBD have been demonstrated in several clinical trials with a low rate (7.1%) of adverse events [3]. Nasopharyngitis, upper respiratory tract infections, arthralgia and arthritis have been described among the most common adverse events while skin reactions have been less recorded [3, 4].
For the first time, we report 4 patients who developed atopic dermatitis after starting therapy with vedolizumab. For each patient a complete evaluation with skin biopsy, prick and patch tests was carried out.
To our knowledge, we describe first cases of atopic dermatitis occurring during VDZ therapy.
We report the cases of 4 patients (2 male and 2 female), who were completely asymptomatic for AD before starting vedolizumab for CU treatment. Those patients had all previously undergone several systemic therapies for the treatment of CU with low or no efficacy.
Two patients had a previous history of atopic-related disease; the family history was positive for atopic-related diseases in 3 patients. Patients’ demographic, clinical and allergological characteristics are reported in Table 1.
Table 1
Demographic, clinical and allergological characteristics of patients
| Case | Sex | Age | History of atopy | No. of VDZ cycle before AD onset | Site of lesions | EASI | IGA | Total IgE [IU/ml] | Family history of atopy | Food/aeroallergens – SPT* | Patch test (T.R.U.E. TEST®) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 71 | Allergic rhinitis | 1 | Neck, face | 3.6 | 2 | 260 | Yes | Grass pollen (++) dermatophaghoides (+) | p-tert-butylphenol formaldehyde resin (+) |
| 2 | M | 33 | Allergic rhinitis | 1 | Back, trunk | 3.2 | 1 | 315 | Yes | Cypress pollen (+) | Negative |
| 3 | F | 40 | No | 1 | Flexor surfaces of limbs | 2.1 | 1 | 302 | No | Negative | Negative |
| 4 | M | 31 | No | 2 | Flexor surfaces of limbs | 2.1 | 1 | 442 | Yes | Negative | Negative |
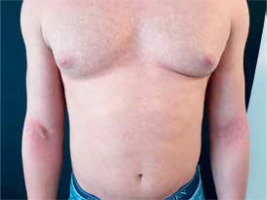
All the patients showed the appearance of symmetrically distributed erythematous plaques in the flexural regions, neck, or back associated with cutaneous xerosis and pruritus after the first or second administration of VDZ (Figure 1). For each patient a complete dermatological and allergological evaluation was carried out. Although only 2 patients had a previous atopic history (allergic rhinitis), the appearance and the location of lesions suggested a clinical diagnosis of adult-onset atopic dermatitis. In order to exclude a drug allergic reaction, a VDZ skin prick test and patch test were performed with negative results. Moreover, the standard patch test (T.R.U.E. TEST® with reading at 72 h) revealed a positive result in 1 case for p-tert-butylphenol formaldehyde resin alone, but this finding did not explain patient cutaneous symptoms. The skin prick tests for aeroallergens show different sensitization profiles in patients who suffer from allergic rhinitis. In every patient a skin biopsy was performed: the histological examination specimen confirmed the clinical diagnosis of eczema showing epidermal spongiosis, mild hyperkeratosis and a lymphohistiocytic infiltrate in the dermis with an increased number of infiltrating eosinophils.
Figure 1
Clinical presentation of the patient (case 3) with symmetrically distributed erythematous papules and plaques on the flexor surfaces of limbs associated with cutaneous xerosis and pruritus
The total serum IgE level was also elevated in all the patients ranging from 260 to 442 international units (IU)/ml (NV < 100 international units (IU)/ml).
Severity of disease was evaluated through EASI (Eczema Activity and Severity Index) [5] and IGA (Investigator Global Assessment) [6] with values ranging from 2.1 to 3.6 and from 1 to 2, respectively. Because the severity of the disease was mild or moderate in all patients, there was no need to discontinue ongoing treatment with VDZ even considering previous treatment failures. As a result, an exclusively topical therapy based on emollients, steroids and/or calcineurin inhibitors was prescribed.
Vedolizumab (VDZ) is a humanized monoclonal antibody, targeting α4 β7 integrin, approved to treat moderate-severe inflammatory adult ulcerative colitis (UC) and adult Crohn’s disease (CD) [1, 2]. The α4 β7 integrin is a transmembrane receptor expressed on the surface of T-lymphocytes that acts as a leukocyte recruiter in the gastrointestinal tract interacting with mucosal addressin cell adhesion molecule-1 (MAdCAM-1) [7]. This molecule is predominantly expressed on endothelium of gut mucosa, and it is upregulated during inflammation processes [8], although some finding suggests a spread of MAdCAM-1 also outside gastrointestinal tract vessels [9]. The efficacy and safety of VDZ in adults with IBD have been demonstrated in several clinical trials [1–4] however a low rate (7.1%) of adverse events is reported in population-based studies [3]. The most common adverse events were infections followed by arthralgia and arthritis as well as skin manifestations. Cases of pruritus, acne, erythema nodosum, psoriasis, dry skin and acute generalized exanthematous pustulosis (AGEP) have already been reported among skin reactions [10–13]. It is entirely unknown what happens to activated T-cells when they are acutely blocked from homing to the gut and if they then have preferential migration to alternative sites, but changes in lymphocyte trafficking during VDZ induction need to be studied and could yield important information on AD appearance mechanisms and risks of therapy. During VDZ therapy activated T-cells are acutely blocked from homing to the gut, but there is no knowledge about a possible preferential migration of these lymphocytes to other body areas, including the skin, during treatment. Changes in lymphocyte trafficking during therapy with VDZ need to be deeply studied and could yield important information on AD onset mechanisms and risks of therapy.
It is also important to emphasise that some drugs approved for the treatment of IBD are also approved or undergoing trials for the treatment of atopic dermatitis [14].
In conclusion, VDZ therapy induced the development of atopic dermatitis in 4 patients described in this article. Some of these patients had no personal or family history of atopic dermatitis or Th2-mediated diseases. Vedolizumab offers a targeted, gut-selective mechanism of action with no clear increased risk of serious adverse events or other complications common to a set of diseases that typically require lifelong therapy. However, it is important to highlight that in our case, the skin manifestations were mild to moderate and did not prompt discontinuation of therapy. Further studies are needed to understand the mechanism underlying the development of atopic dermatitis.








