INTRODUCTION
In recent years, specific allergen immunotherapy (AIT) has emerged as a key therapeutic method for treating allergic diseases. Its efficacy and safety have been demonstrated in numerous clinical studies [1, 2], and the preventive aspect of this therapy has become a crucial factor influencing the expansion of its indications. Immunotherapy not only alleviates allergic symptoms but also modifies the natural course of allergic diseases, potentially reducing the risk of developing new allergies and asthma [3]. This has firmly positioned AIT as a cornerstone of modern allergy management.
However, despite the promising benefits, the implementation of AIT is often hindered by contraindications that are not always backed by robust scientific evidence but are instead based on long-standing assumptions. These limitations can complicate the determination of eligibility and deny many patients access to this effective treatment. In particular, the approach to autoimmune diseases as a contraindication is the subject of ongoing controversy.
This issue is not uncommon, as autoimmune and allergic diseases often coexist. Over the last three to four decades, many regions have faced an ‘allergy epidemic’ [4], while a simultaneous increase in the incidence rates of several autoimmune conditions has been noted [5]. Recent studies have shown that the long-term risks of these disorders are significantly higher in patients with allergic diseases [6]. Therefore, understanding the complex relationship between autoimmune conditions, allergic diseases, and allergen immunotherapy is essential for optimizing patient outcomes and expanding the safe application of this therapy.
HISTORICAL CONTEXT AND THEORETICAL PARADIGMS
The discussion on the impact of AIT on the development and progression of autoimmune diseases began in the 1980s. Doubts arose after the first report describing cases of autoimmune disease onset following the initiation of immunotherapy for inhalant allergens or Hymenoptera venom during several preceding years [7]. This notion was supported by the theoretical framework of T helper type 1 (Th1) and T helper type 2 (Th2) responses, which suggests that the immune system in allergic patients is skewed towards Th2 predominance, whereas in those with autoimmune diseases, it is inclined towards Th1 prevalence. Following this logic, it was implied that allergen immunotherapy, by inhibiting the Th2-dependent response, could potentially increase the risk or exacerbate the course of autoimmune diseases.
In the subsequent years, additional cases of autoimmune diseases occurring during immunotherapy were reported. These included conditions such as Sjögren’s syndrome [8], multiple sclerosis [9], localized scleroderma [10], rheumatoid arthritis [11], recurrent pericarditis [12], and vasculitis [7, 13–17]. As a result, numerous scientific societies and international guidelines, guided by the precautionary principle, have listed autoimmune diseases as relative or absolute contraindications to allergen immunotherapy, despite the lack of any data confirming possible AIT-related causality in those cases.
Additionally, in the last decade, the role of vaccine adjuvants has become a focus of intense discussion. Studies analyzing this topic emphasize that, despite the over 200-year history of vaccination use, no data have yet confirmed the theory that vaccine adjuvants contribute to the development of autoimmune diseases. While some adjuvants, due to their mechanisms of action, could theoretically increase this risk, evidence from large-scale studies on vaccines indicates low rates of such disorders [18], suggesting a minimal link between adjuvants and autoimmunity. Additionally, the multifactorial nature of these conditions is underscored, including the roles of genetic predisposition and environmental influences. This comprehensive view underscores the importance of continuing to monitor and research vaccine safety while acknowledging the broader context of autoimmune disease aetiology.
CURRENT STATE OF KNOWLEDGE
EVIDENCE FROM CASE REPORTS
The earliest cases linking autoimmune diseases with AIT date back to the 1970s. Vasculitis was the most commonly reported reaction in these case reports, and it was also the most frequently reproducible upon repeated exposure to AIT.
In a pivotal 1980 publication, Phanuphak and Kohler reported that out of 20 consecutive cases of polyarteritis nodosa diagnosed in their centre, 6 patients developed the first symptoms within 3 months of starting allergen immunotherapy [7]. The authors noted that respiratory symptoms determining eligibility for immunotherapy could have been misinterpreted as signs of existing vasculitis rather than allergies. However, they emphasized that in 3 patients, rhinitis and asthma symptoms preceded immunotherapy by 8 to 22 years, ruling out a misdiagnosis of vasculitis. The authors concluded that the injected antigens were indirectly responsible for systemic arteritis, likely by inducing antibodies with cross-reactivity to endogenous host antigens. Evidence of circulating immune complexes was found in five of the cases.
Subsequent case reports also predominantly featured vasculitis following allergen injections, showing both a short-term temporal association and reproducibility [13–16]. Given that the suggested mechanism of vasculitis after allergen injection is a type III hypersensitivity response involving circulating antibody-allergen immune complexes, these reactions were not considered as typical autoimmune disorders. Their mechanism is more akin to serum sickness from horse serum than a primary autoimmune disease.
One noteworthy point brought up while discussing the case reports mentioned above is that many of these reports were relatively old, and the allergen extracts used for AIT at that time were not well purified [19]. This lack of purification might have led to immune complex formation, which could have contributed to the development of the diseases observed.
Further cases of autoimmune diseases emerging in correlation with AIT require thorough evaluation. A significant portion of the available evidence can be considered circumstantial, such as a report of pericarditis associated with subcutaneous immunotherapy (SCIT) [2], in which an immunologic mechanism was suggested, but not definitively proven. Nakajima et al. described a case of systemic sclerosis that appeared after administering an influenza vaccine together with an allergen vaccine [9]. However, this case is not definitive, as another report described increased clinical activity of the disease and magnetic resonance imaging findings in a patient with scleroderma following only influenza vaccination [20]. While Maciel and Morfin reported a case of localized scleroderma in connection with immunotherapy [10], it is important to note that their patient had a positive family history of systemic sclerosis. Turkcapar et al. documented Sjögren’s syndrome [8], with symptoms appearing about 5 months after starting SCIT. However, after AIT was stopped, there were no changes in symptoms and laboratory findings during the first year of follow-up, making the relationship with AIT less convincing. Ghoreschi et al. highlighted the manifestation of rheumatoid arthritis (RA) during SCIT with bee venom [11], but anti-cyclic citrullinated peptide (anti-CCP) results turned out to be positive prior to starting AIT (although at a significantly lower level than after SCIT injections), indicating that the patient was at increased risk of RA. Thus, AIT might have acted as a trigger of RA in the immunologically predisposed patient.
Only a few reports on the safety of immunotherapy in patients with autoimmune diseases are available, as allergists are often hesitant to evaluate such patients as eligible for this treatment due to potential perceived risks. One notable example is a patient with systemic lupus erythematosus (SLE) who underwent venom immunotherapy for Hymenoptera venom allergy [21]. Remarkably, the patient’s autoimmune disease remained in remission throughout a one-year follow-up period during the immunotherapy.
A two-year observational case series was conducted to examine the impact of AIT on the activity of concurrent rheumatic autoimmune diseases (RAD) and its effectiveness in patients with allergic rhinitis complicated by RAD [22]. In a particular centre, 8 patients undergoing AIT with coexisting RAD were identified and monitored over 2 years to assess RAD symptoms and activity levels. Most patients with RAD continued AIT without experiencing an exacerbation of their rheumatoid symptoms. Only 3 patients showed a temporary increase in RAD activity, but the attending physician ruled out a causal relationship with immunotherapy. This was either because AIT had already been discontinued, or due to other clear factors, such as environmental stress, changes in biological treatment, or pre-existing symptoms unrelated to AIT.
The incidence of autoimmune conditions linked to AIT appears to be low, especially considering that immunotherapy has been in use for over a century and patients typically undergo long-term treatment. It is likely that many cases of autoimmune diseases associated with AIT have not been reported, resulting in an underestimation of the actual number.
EVIDENCE FROM OBSERVATIONAL STUDIES
Further evidence on the association between AIT and autoimmune diseases comes from observational studies. Linneberg et al. conducted the first epidemiological study to investigate the potential risk of autoimmune diseases and ischemic heart disease in patients undergoing allergen immunotherapy [23]. The study found a slightly lower risk of autoimmune diseases in patients with allergic rhinitis receiving AIT compared to those receiving pharmacological allergy treatment. However, it is crucial to note that this risk estimate was assessed in a general population and not those with a predisposition to autoimmune diseases.
Bozek et al. conducted a long-term observational case-control study examining the safety of AIT in patients allergic to house dust mites and pollen concerning the development of neoplasia and autoimmune conditions [24]. The prevalence of autoimmune diseases in the AIT treatment groups was slightly lower than in the control group, but the differences were not significant. The treatment groups had 34 new instances of autoimmune diseases compared to 41 in the control group. The conditions observed included Hashimoto’s thyroiditis, lupus erythematosus, chronic ulcerative colitis, and vitiligo. The study concluded that AIT did not influence the incidence of autoimmune diseases, and the occurrence rates were comparable to those in the control group. Laboratory results supported these observations.
It is important to recognize the limitations of the studies mentioned above. Some of the negative effects observed could be explained by confounding factors, such as the possibility that patients receiving AIT are generally better controlled and receive more thorough care than those receiving conventional allergy treatment, which requires fewer specialist visits. This underscores the need for more rigorously controlled studies to provide unbiased estimates.
EVIDENCE FROM CONTROLLED STUDIES
Presently, there are no published studies on the risks associated with AIT in allergic patients with underlying autoimmune diseases. Ethical and practical challenges complicate such research. Similarly, there is a lack of data on the influence of these conditions on the efficacy or safety of AIT, e.g. whether having an autoimmune disease predisposes patients to severe side effects during AIT. However, potential opportunities for advancing our understanding in this area lie in large-scale, multi-centre European real-life observational studies, which could help to clarify these risks and guide clinical practice.
REVIEW OF INTERNATIONAL SCIENTIFIC SOCIETY GUIDELINES
The lack of clinical studies on contraindications to immunotherapy often causes international guidelines to rely primarily on expert opinions, replicate older guidelines, or remain vague on specific topics. In the case of autoimmune diseases, many issues have not reached a consensus and thus are rarely discussed in detail (Figure 1). Autoimmune diseases are frequently considered as relative contraindications [25–31], leaving the decision to determine a patient’s eligibility for immunotherapy at the discretion of the physician, based on the risk-to-benefit ratio [26, 32–34]. Consequently, in the absence of clear guidelines, the decision to initiate immunotherapy can vary depending on the practitioner.
Figure 1
Global variations in approach to autoimmune diseases as a contraindication to allergen immunotherapy, as seen in international recommendations
American Academy of Allergy, Asthma and Immunology (AAAAI), Mexican College of Clinical Immunology and Allergy (CMICA), Danish Society for Allergology (DSA), British Society for Allergy and Clinical Immunology (BSACI), British Medical Association (BMA), Spanish Society of Allergology and Clinical Immunology (SEAIC), Norwegian Society for Allergology and Immunopathology (NSAI), European Academy of Allergy and Clinical Immunology (EAACI), Svenska Föreningen för Allergologi (SFFA), Polskie Towarzystwo Alergologiczne (PTA),Italian Society of Pediatric Allergy and Immunology (SIAIP), Chinese Society of Allergology (CSA), Japanese Society of Allergology (JSA), Korean Academy of Asthma, Allergy and Clinical Immunology (KAAACI), German Society for Allergology and Clinical Immunology (DGAKI), Australasian Society of Clinical Immunology and Allergy (ASCIA).
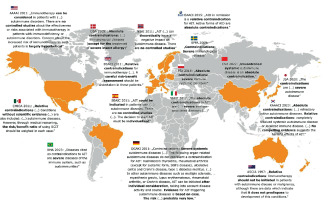
This variability is highlighted by a survey conducted by the European Academy of Allergy and Clinical Immunology, which was targeted at members who practice immunotherapy [35]. The survey gathered responses about treating patients with immunotherapy in real-life conditions, who theoretically had contraindications to such therapy. According to the survey, 29.4% of the responding physicians had experience treating patients with autoimmune diseases. Physicians with more experience tended to report fewer issues and were less likely to view these conditions as an absolute contraindication compared to less experienced physicians. Among the patients with autoimmune diseases, 76.4% experienced no problems during immunotherapy, suggesting that with appropriate precautions, AIT can be safely administered to these patients.
Most guidelines state that active autoimmune diseases are an absolute contraindication for AIT [27–31, 36–41]. For stable, well-controlled organ-specific autoimmune diseases, the decision to initiate AIT often involves careful consideration on a case-by-case basis, with many guidelines allowing AIT under close monitoring. This approach is often easier to implement in the case of venom immunotherapy (VIT), for which autoimmune diseases are considered a relative contraindication in most guidelines due to the life-threatening risk posed by Hymenoptera venom allergies [27, 42]. There is a consistent emphasis on the lack of controlled studies specifically addressing the risks associated with AIT in patients with autoimmune diseases. It would be beneficial for guidelines to distinguish between the application of AIT in patients with organ-specific autoimmune disorders (such as Hashimoto’s thyroiditis or RA) and those with systemic autoimmune disorders, as seen in the German guidelines [30] (Table 1).
Table 1
Recommendations for AIT in patients with autoimmune diseases: position statement of the Immunotherapy Section of the Polish Society of Allergology on Allergen-Specific Immunotherapy (ASIT) in patients at risk of or diagnosed with autoimmune diseases [44]
SUMMARY
Currently, the only causal treatment method for allergies is AIT. Although the classical protocol for AIT, introduced in 1911 [43], has not changed drastically, immunotherapy has undergone a significant evolution since then to become a milestone in allergy treatment. The development of new strategies aimed at enhancing the safety and efficacy of AIT has facilitated its increasingly widespread use. Recent advancements include new administration routes, novel adjuvants, the use of recombinant allergens, and the combination of allergens with monoclonal antibodies or immune modifiers. These innovations are expanding the indications for this therapy, offering hope for broader applicability and improved patient outcomes.
Despite these advancements, international guidelines often lag behind the rapid developments in evidence-based medicine (EBM). A significant gap remains in the area of contraindications to immunotherapy, which are frequently based on outdated case reports. These historical guidelines were formulated when allergen vaccines differed significantly from those used today, and when recommendations were primarily based on expert opinions, anecdotal cases, and theoretical assumptions rather than robust EBM principles.
This review highlights the substantial gaps and controversies in the understanding of autoimmune diseases as contraindications to allergen immunotherapy. It underscores the urgent need for further research to provide solid evidence that will enable safe determination of eligibility for this effective therapy for all patients. Addressing these gaps through rigorous, well-designed studies will ensure that AIT can be administered safely and effectively to patients with autoimmune conditions, ultimately enhancing their quality of life and therapeutic outcomes.







