CASE REPORT
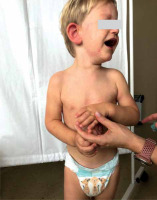
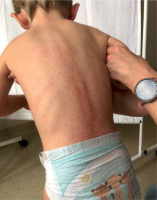
A 2-year-old Caucasian boy with suspected lactose intolerance presented with an itchy rash on his face, ears, neck, trunk and limbs (Figures 1 and 2). A few days earlier he was admitted to the emergency department with signs of a viral upper airway infection, rhinitis and fever. There were no signs of rash, cough or shortness of breath at admission. Ibuprofen, xylometazoline and cetirizine were prescribed and he was discharged later that day. After 2 days, xylometazoline was replaced by a combined drug containing both xylometazoline and dexpanthenol since the former drug finished up. Several hours later the boy developed an itchy widespread rash which led to sleep deprivation on the following night. As the rash got worse, the caregivers decided to seek medical advice at a general practitioner’s office. At admission there were no signs of shortness of breath, swallowing or diarrhea and his condition was stable. The infectious cause of the rash was eliminated in the diagnostic process. The mother had not implemented any dietary changes lately nor had she changed washing agents. In the absence of any other possible cause of allergy, the allergic response to dexpanthenol was diagnosed. The caregivers were told to withdraw all drugs except cetirizine. After a few days of therapy, the rash was gone.
DISCUSSION
DEXPANTHENOL AND ITS USE
Dexpanthenol is an alcohol analog of pantothenic acid (vitamin B5) and is broadly used in skincare and cosmetic products because of its moisturizing properties. When applied topically, it is rapidly converted to pantothenic acid [1], which is a component of coenzyme A involved in various metabolic processes, including the synthesis of fatty acids and the conversion of carbohydrates into energy [1, 2]. Due to the fact that nasal decongestants like xylometazoline can sometimes cause dryness or irritation in the nasal passages, dexpanthenol is included in some medications to counteract these effects as it has a positive impact on all three phases of tissue repair, comprises protection from infection and free radicals, modulation of inflammation, support of cell proliferation, and acceleration of migration [3].
Although considered relatively safe, with no overdose cases reported, the allergic potential of dexpanthenol has been proven and is believed to have a microsomal-dependent background [4].
ALLERGIC POTENTIAL OF DEXPANTHENOL
There are several studies showing the allergic potential after topical administration of medications and cosmetic products containing either panthenol or dexpanthenol.
Firstly, Schmid-Grendelmeier et al. reported 7 cases with an allergic response to panthenol and reviewed the literature to find 40 more cases before 1995 [5]. Then Clerens et al. identified 23 out of 3301 patients to have a positive reaction to panthenol in the patch test. The developed symptoms of atopic dermatitis localized mostly on the face and hands, less frequently on the trunk, legs and feet [6]. Fernandes et al. tested a group of 2171 patients out of which 26 patients developed allergic reactions to dexpanthenol [7]. In both studies the percentage of patients developing allergic contact dermatitis was similar, 0.7% and 0.89%, respectively, which proves it to be rare and therefore easy to overlook.
There are two cases of allergic response to dexpanthenol in children. One of them reports an 11-year-old girl who developed allergic contact dermatitis after make-up application [8], the other one, an 8-year-old girl presented with pruriginous pustular lesions on her face and neck 2 days after administration of a cosmetic product containing dexpanthenol [9]. What differs our case from these two is that in both former cases the rash appeared in the place of dexpanthenol application, whereas our patient developed a widespread rash on his skin after using a nasal spray into the nasal passages.
ALLERGIC REACTIONS TO NON-STEROIDAL ANTI-INFLAMMATORY DRUGS
The allergic potential of non-steroidal anti-inflammatory drugs (NSAIDs) is well known and has been reported in many studies. We decided to mention this subject since ibuprofen was used in the patient’s therapy and according to the American College of Allergy, Asthma and Immunology, NSAIDs are classified as one of the most common drugs to have allergic potential. That is why, after the comparison of our case with the literature, we amplified medical interview with our patient’s mother to find any evidence of any previous drug allergy to ibuprofen. The feedback we received contained no relevant information about any previous incidents appearing after NSAIDs administration, nor later in a 3-month follow-up in the period from our medical intervention until the publishing of this case despite being frequently used in our patient because of his infections in the flu season.
Due to the fact that there are several agents to have lactose as one of excipients (e.g. Ibuprofen-Pabi) and that our patient also has suspected lactose intolerance, we decided to check if an accidental lactose intake could be the reason of rash in this case. We found that no pharmaceutics containing both ibuprofen and lactose were administered to our patient at that time, so we excluded this possibility.
THE LIMITATIONS OF OUR CASE
Although we did our best to exclude any other possible cause of allergy and there is an unprecedented timeline coincidence between the signs of allergy appearance, the change of the drug used in the therapy and allergic potential of dexpanthenol, the limitations of our case report need to be underlined. Firstly, we could not run any patch test to confirm the allergy. We also did not receive the mother’s approval to run a provocative test. Secondly, we have not received any information about later re-exposure. Finally, the allergic potential of other excipients of the drug cannot be excluded. Nevertheless, we believe that dexpanthenol hypersensitivity was the most probable cause of symptoms in this case.
CONCLUSIONS
Although oversensitivity to dexpanthenol seems to be rare, it should always be taken into consideration when dealing with a patient developing a widespread rash with unknown cause. Dexpanthenol is a common agent in many cosmetic and medical products, hence the allergic response may appear at all ages, both in females and males. Even if a severe, life-threatening allergic response has not been reported, it may still cause sleep deprivation and visual changes that need to be taken care of to improve the quality of life and provide the best possible medical care to affected individuals.