Introduction
Colorectal cancer (CRC) is the third most common and the second most deadly cancer worldwide, accounting for nearly 2 million new cancer cases and almost one million deaths worldwide in 2020 [1, 2]. Pathomorphological evaluation of postoperative material is routinely used in clinical practice, allowing for determination of CRC’s features related to its invasion within the intestinal wall (T) as well as nodal (N) and distant metastasis (M) [3]. These classical morphological parameters are still the major basis for risk stratification and selection between different postoperative treatment options. Contemporary CRC treatment is realized as a personalized therapy and is based on the selection of neo- and/or adjuvant therapy in reference to the individual molecular cancer subtype, stage of disease, and general condition of the patient [2, 4, 5]. Therefore, it is necessary to identify and validate new proteins useful as prognostic and/or predictive markers that can be easily adopted for a routine immunohistochemical evaluation of postoperative CRC tissues.
DNA polymerase delta (Polδ) is an enzyme complex responsible for complementary synthesis of the lagging strand during DNA replication, and it participates, alongside DNA polymerase epsilon (Polε), in the replication of the leading DNA strand [6]. Polδ and Polε are heterotetrametric complexes, and their catalytic subunits are DNA polymerase delta 1 catalytic subunit (POLD1) and DNA polymerase epsilon, catalytic subunit (POLE), respectively [7]. Polymerase delta 1 catalytic subunit and POLE possess DNA polymerase and 3’–5’ exonuclease activities, and they serve in the mechanisms of DNA repair [8]. Genetic alterations of exonuclease domains of POLD1 and POLE may affect their DNA proofreading ability and are considered factors in the development of several human neoplastic diseases including hereditary and sporadic colorectal tumours [8–10]. Pathologic variants of POLD1 and POLE can result in deficient DNA replication and repair predisposing to colorectal polyposis and subsequent neoplastic transformation [11, 12]. The studies based on colon cancer cell lines HCT116 and SW48 as well as sporadic CRCs indicate that POLD1 gene mutation within its exonuclease domain impairs Polδ function resulting in accumulated mismatches of DNA base paring [13]. Colorectal tumours that develop on the basis of polymerase proofreading deficiency or impaired mismatch repair system are characterized by higher mutation burden and higher infiltration by neoantigen-specific, tumour-infiltrating T-cells, predisposing those tumours for immunotherapy [14]. This suggests that POLD1 and POLE mutations can be predictive markers useful in identifying gastrointestinal tumours that are sensitive for treatment with immune checkpoint inhibitors [15, 16]. However, POLD1 mutations in sporadic CRC are rarer than those in POLE, and no individual mutational signature has been associated with POLD1 deficiency in microsatellite stable CRC [17], questioning the predictive significance of POLD1 genetic alterations in most molecular subtypes of sporadic CRCs.
While the genetic alterations of POLD1 in CRC get most of the attention, the level of POLD1 expression in postoperative material of CRC patients, and its association with the tumour progression and patients’ outcomes have not been extensively studied, and the usefulness of POLD1 protein or mRNA level as a prognostic marker in CRC remains unclear. Therefore, the purpose of this study was to determine the expression levels of POLD1 protein in the tumour and matched normal tissues collected from CRC patients, subjecting tissue sections to a standard, cheap, and easy to adopt in any pathological laboratory method such as immunohistochemistry (IHC). In addition, POLD1 mRNA expression data were extracted from The Cancer Genome Atlas (TCGA) CRC datasets and analysed.
Material and methods
Ethical statements
The study was approved by the Bioethical Commission of the University of Warmia and Mazury, Olsztyn, Poland (no. 40/2010 and 21/2017) and was conducted according to the guidelines of the Declaration of Helsinki (1975, as revised in 2000). Written informed consent was obtained from each patient included in this study.
Patients and collection of tissue samples
The colorectal cancer study group consisted of 78 patients (44 males and 34 females; mean age 67.5 years old, ranging 33–90). The tissue samples were collected from patients who underwent open surgery resection large interstine due to the CRC tumour. Only patients with histologically confirmed CRC (colonoscopic biopsy) and complete clinicopathological data (including computed tomography examination of abdominal and pelvis cavity) were qualified to the study. Patients with neoadjuvant chemotherapy and/or radiotherapy as well as patients with polyps in the colorectal region associated with the cancer were excluded from the analysis. The demographic and clinicopathological data of CRC patients are presented in Table 1. Shortly after resection 2 tissue specimens (whole wall of intestine, each 1–2 cm in size) were collected: one directly from the tumour and one from a distant, morphologically unchanged part of the large intestine. The samples collected from postoperative material were fixed in 4% buffered (pH 7.4) solution of paraformaldehyde. For clinical use, the cancer characteristic and staging according to the 8th edition of American Joint Committee on Cancer [5] were evaluated (pathologist H.M). Patients were followed-up, and the average time of observation was 52.2 months.
Immunohistochemistry and evaluation of immunohistochemical reactions
All sections of CRC lesions and matched normal, morphologically unchanged large intestine were subjected to immunohistochemical staining with the use of antibodies directed against POLD1 protein (HPA046524; Merck KGaA, Darmstadt, Germany) using a previously described protocol [18]. The polymerase delta 1 catalytic subunit nuclear immunoexpression levels were evaluated in CRC tumours and epithelial cells (enterocytes) of the matching large intestine according to the immunoreactive score system of Remmele and Stegner (IRS) [7] as previously described by Godlewski et al. [18]. The colorectal cancer patients were divided based on the median immunoexpression score in CRC cells (median IRS = 6) into 2 groups described as ‘low’, and ‘high’ POLD1 immunoexpression.
Bioinformatic analysis of The Cancer Genome Atlas datasets
Polymerase delta 1 catalytic subunit RNA-Seq gene expression data of 599 samples derived from primary CRC sites were extracted from the TCGA-COAD and TCGA-READ datasets [19] (accession date: 13 May 2023) and subjected to further bioinformatical analysis. Patients without complete demographic (sex, age) or staging (tumour-node-metastasis – TNM) data were excluded from the analysis. Follow-up data for overall survival (OS) and disease-free survival (DFS) of the patients were collected for 599 CRC cases from cBioPortal (accession date: 17 March 2023) [20]. Differential gene expression analysis was performed using DESeq2 [8] with absolute value of log2FoldChange ≥ 0.5 and p-value < 0.05 as the cut-off. Based on the median of normalized gene expression values of the POLD1 gene the entire dataset was divided into 2 groups classified as ‘low’, and ‘high’ POLD1 mRNA expression.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism software, version 6.07 (GraphPad, La Jolla, CA, USA) and Statistica version 13 (TIBCO Software Inc., Palo Alto, CA, USA) software. The significance of differences between expression levels of POLD1 proteins in CRC cells and normal intestine epithelial cells were tested by the Wilcoxon matched-pairs signed-rank test. The ratios of POLD1 scores of paired cancer cells and epithelial cells of normal intestine sections were calculated. Correlations of POLD1 mRNA and protein expression levels with demographic and clinicopathological data were evaluated using Fisher’s exact test. The univariate associations of POLD1 mRNA and POLD1 protein levels or demographic and clinicopathological data with patients’ OS or DFS were plotted using the Kaplan-Meier method, and the survival differences between the patient cohorts were assessed by the log-rank test. Differences were considered statistically significant for a p-value lower than 0.05
Results
Polymerase delta 1 catalytic subunit protein was found in the nuclei of CRC cells (Figs. 1 A, B) and epithelial cells of unchanged mucosa of the large intestine (Figs. 1 C, D). In addition to CRC or epithelial cells, POLD1 immunoreactivity was noticeable in the nuclei of some stromal cells. However, when compared to the tumour cells (Figs. 1 A, B) or epithelial cells of intestinal mucosa (Figs. 1 C, D), the density and staining intensity of POLD1-positive cells in the stroma were low. Polymerase delta 1 catalytic subunit immunoexpression in levels CRC sections were heterogenous, and their score ranged from IRS = 2 to IRS = 12. Polymerase delta 1 catalytic subunit 1 immunoreactivity levels were equal to or lower than the median value (IRS = 6) in 47/78 (60.3%) and higher than the median IRS in 31/78 (39.7%) tumour sections (Fig. 2 A). Polymerase delta 1 catalytic subunit immunoexpression was decreased in 33 (42.3%) and increased in 20 (25.6%) tumours when compared to the POLD1 immunostaining in the epithelial cells of the matched section of noncancerous large intestine. In the sections derived from 25 CRC patients (32.1%), the CRC cells and matched epithelial cells did not differ in regard to the level of POLD1 protein expression. The average immunoreactivity level of POLD 1 was significantly decreased in the nuclei of CRC cells as compared to the epithelial cells of unchanged large intestine mucosa (IRS 6.40 ±0.33 and IRS 7.06 ±0.33, respectively; p = 0.0259) (Fig. 2 B).
Fig. 1
Immunohistochemical staining of DNA polymerase delta 1 catalytic subunit (POLD1) protein in representative sections of the tumour and large intestine tissues of a colorectal cancer (CRC) patient. Nuclear immunoreactivity of POLD1 in CRC cells (A, B), nuclear immunoreactivity of POLD1 in epithelial cells of the matched, unchanged mucosa of the large intestine (C, D). Magnification 200× (A, C), 400× (B, D)
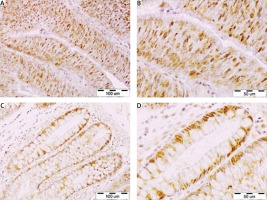
Fig. 2
Evaluation of DNA polymerase delta 1, catalytic subunit (POLD1) immunoexpression in the colorectal cancer (CRC) cells and epithelial cells of morphologically unchanged large intestine by immunohistochemistry. Polymerase delta 1, catalytic subunit nuclear immunoreactivity in the tumor sections of individual patients with CRC are shown. Grey bars represent patients revealing low POLD1 immunoreactivity, black bars represent patients with high levels of POLD1 immunoreactivity (A), the average nuclear immunoreactivity of POLD1 in the tumor cells are shown in relation to POLD1 levels in the epithelial cells of morphologically unchanged large intestine (B). Data are presented as the means ±SEMs (n = 78)
CRC – colorectal cancer, IRS – immunoreactive score of Remmele and Stegner, POLD1 – polymerase delta 1 catalytic subunit

Polymerase delta 1 catalytic subunit expression levels in the tumour cells did not correlate with demographic (sex, age) and clinicopathological (tumour localization, primary tumour status, regional lymph nodes status, presence of distant metastasis, and TNM stage) data of CRC patients (Table 1, left panel).
Table 1
No associations between clinicopathological features of colorectal cancer patients and DNA polymerase delta 1 catalytic subunit (POLD1) nuclear immunoreactivity (as determined by immunohistochemistry; n = 78) or POLD1 mRNA expression levels (as extracted from The Cancer Genome Atlas [TCGA] datasets TCGA-COAD and TCGA-READ; n = 599)
Similarly, POLD1 mRNA expression data extracted from TCGA-COAD and TCGA-READ datasets did not correlate with the demographic characteristics of the patients and the progression of CRC (Table 1, right panel) and did not reveal significant associations between the levels of POLD1 mRNA and OS or DFS of the CRC patients (Figs. 3 A, B).
Consequently, immunoexpression of POLD1 in CRC cells did not correlate with CRC patients’ outcomes (p = 0.1856) (Fig. 3 C). Among the demographic and clinicopathological data subjected to survival analysis, the higher status of primary tumour (HR = 2.14, p = 0.0477), N1 or N2 status of nodal metastasis (HR =2.07; p = 0.0371), presence of distant metastases (HR = 3.12; p = 0.0007), and higher TNM stage (HR = 2.51; p = 0.0089) correlated with worse prognosis in CRC patients (Figs. 3 G–J) while the other parameters such as sex, age, tumour localization did not reveal prognostic significance (Figs. 3 D–F).
Fig. 3
Kaplan-Meier diagrams presenting the survival of patients with colorectal cancer (CRC) regarding the expression levels of the polymerase delta 1 catalytic subunit (POLD1) (A–C) and demographic or clinicopathological characteristics of the patients (D–J): overall survival (A) and disease-free survival (B) of CRC patients regarding POLD1 mRNA expression levels (The Cancer Genome Atlas datasets; n = 599). Overall survival of CRC patients (n = 78) regarding the nuclear POLD1 immunoreactivity in cancer cells (C), sex (D), age (E), tumour localization (F), primary tumour status (G), regional lymph node status (H) Distant metastases (I), and tumour-node-metastasis stage (J). Significant p-values (< 0.05) for corresponding log-rank tests are given in bold

Discussion
Most of the previous studies explored the prevalence of pathogenic POLD1 variants and the association of genetic alterations with CRC patients’ survival and therapy effectiveness. Our studies aimed to investigate POLD1 immunoexpression in postoperative CRC material, and to test the association between protein levels in reference to the clinicopathological data and patients’ outcomes. The immunoexpression levels of POLD1 in our analysed CRC specimens were heterogenous, allowing the cohort to be divided into the 2 subgroups that were then analysed for correlations with disease progression and patients’ outcomes. However, we did not find any significant association between the POLD1 immunoreactivity and clinicopathological data of the patients. Moreover, the survival analysis carried out in the cohort of 78 patients and 2 TCGA-derived datasets of 599 patients did not reveal any prognostic significance of POLD1 expression in CRC.
A previous study investigating POLD1 immunoexpression in a cohort of 1069 CRC patients from the Middle East revealed significant correlations between low POLD1 levels and mucinous histological type of the tumour, higher primary tumour status, and higher tumour stage, suggesting a suppressive role of POLD1 protein in the progression of CRC [21]. However, the latter study also failed to disclose significant associations between the POLD1 immunoexpression and patients’ outcomes [21]. Interestingly, in a recent study by Kim et al. [22] using a mouse model of colon dysplasia and HCT116 and SW480 cell lines, it was shown that the expression of POLD1 in CRC can be controlled via the β-catenin/c-MYC/E2F1 axis [22]. Moreover, inhibition of the β-catenin/c-MYC/E2F1 axis resulted in decreased POLD1 expression followed by increased DNA replication stress, DNA damage response, apoptosis, and overall repression of the tumour growth. Correlations between POLD1 and c-MYC as well as observed results of POLD1 reduction showed that this enzyme was essential for CRC tumourigenesis. Indeed, POLD1 activity decrease both tumour mutational burden and immunogenicity, maintaining genomic integrity of cancer cells above the critical level [23]. Therefore, both low POLD1 immunoexpression level and/or harbouring of POLD1 exonuclease domain mutations shall be considered as part of the signature that potentially correlates with longer survival and benefits from treatment with immune checkpoint inhibitors such as those targeting programmed death-1 (PD-1) and its ligand (PD-L1) [5, 16, 24].
Polymerase delta 1 catalytic subunit and POLE are the catalytic subunits of Polδ and Polε, with DNA polymerase and proof-reading exonuclease activities that are essential for DNA replication and maintenance of cellular genetic homeostasis [17]. Germline and somatic pathogenic variants of POLD1 and POLE with altered proof-reading capabilities have been demonstrated as driver mutations predisposing to the development of hypermutated but microsatellite stable CRC [12, 25]. Interestingly, the genetic alterations of POLE exonuclease domain have been reported in both familial and sporadic CRC, while there is little evidence of equivalent POLD1 somatic mutations [11, 17]. Despite much reported evidence for somatic POLD1 mutations predisposing to develop hypermutated spora- dic and colitis-associated CRC, most of the studied POLD1 alterations were associated with hereditary colorectal syndromes [25, 26]. Mutation status of the POLD1 gene has recently been proposed as a promising predictive marker in CRC [15]. Analysis of genomic data from TCGA dataset including 2674 CRC patients revealed that POLD1/POLE mutations were present in 7.37% of tumours and that the presence of alterations can have predictive significance for positive immune checkpoint inhibitor therapy outcomes [16]. However, the results of our study suggest that the level of POLD1 expression, unlike its genetic status, has no prognostic significance in CRC.
The expression levels of POLD1 and its prognostic or predictive significance have been studied only in several human solid cancers. The study carried out on 432 endometrial carcinoma specimens revealed an association between elevated POLD1 immunoexpression and higher tumour grade [27]. Increased POLD1 expression was shown in breast cancer tissues and breast cancer cell line MCF-7, and this alteration could be at least partially attributed to decreased p53 activity in this tumour [28]. Another study on this tumour revealed that elevated expression of POLD1 correlates with metastasis to the lymph node, higher tumour grade, negative p53 immunostaining, and higher Ki-67 index [29]. The same study revealed prognostic significance of POLD1 in breast cancer while its levels correlated with worse DFS and incidence of triple-negative tumours [29]. In addition, experiments carried out in triple-negative breast cancer cell line MDA-MB-231 demonstrated antiapoptotic properties of POLD1 overexpression in these cells, suggesting that POLD1 could be considered as a potential therapeutic target in breast cancer [30]. Microarray profiling of non-small cell lung cancer specimens revealed that altered POLD1 expression can be a part of the signature associated with the development of lung cancer from chronic obstructive pulmonary disease. Elevated expression of POLD1 was shown to correlate with poor prognosis of lung adenocarcinoma [31]. Zhao et al. [32] applied bioinformatical analysis and IHC to investigate the role of Polδ family members in hepatocellular carcinoma, showing that elevated expression of POLD1 is associated with worse prognosis and development of an immunosuppressive tumour microenvironment. These findings were in line with another studies on hepatocellular carcinoma [33, 34], which documented significant associations between the expression levels of Polδ subunits, in particular POLD1, and patients’ outcomes, tumour infiltration by immune cells, and genomic alterations of the tumour. While most studies suggest an oncogenic character of POLD1 in human malignancies, our previous study on clear cell renal cell carcinoma showed that higher levels of POLD1 protein were associated with longer OS of the patients. The association between the disease progression and decreased POLD1 immunoexpression was also found in a group of 300 papillary thyroid carcinoma patients [35]. The results of the above studies suggest that the potential oncogenic or tumour suppressive role of POLD 1 and its prognostic or predictive significance can be a tumour-specific feature, rationalizing the present and future studies on POLD1 immunoexpression in various types of human cancers.
Conclusions
The present study was limited to immunohistochemical assay only due to routine application of this method in postoperative evaluation of patients with various neoplasms. Our analysis revealed that the immunoexpression of POLD1 is altered in CRC cells, but the protein levels did not correlate with the progression of the disease and patients’ outcomes. This observation was supported by the analysis of POLD1 mRNA expression in CRC that used TCGA datasets. While the genetic status of the POLD1 gene is being investigated for its prognostic or predictive significance in CRC, the results of our study do not support the usefulness of POLD1 expression levels as a prognostic marker in CRC.








