Introduction
Multiple myeloma (MM) is a blood malignancy characterized by the accumulation of monoclonal plasma cells in the bone marrow and, rarely, tumours in other tissues. The most common symptoms of MM are known as CRAB: hypercalcaemia, renal insufficiency, anaemia, and osteolytic bone lesions. According to Global Cancer Statistics 2020 (GLOBCAN2020), MM was the second most prevalent blood cancer worldwide in 2020, with an estimated morbidity of 176,404 and mortality of 117,077 people [1]. Multiple myeloma remains incurable despite the recent development of novel medications such as proteasome inhibitors, immunomodulatory drugs (IMiDs), monoclonal antibodies, and more sophisticated immunotherapies [2, 3]. Current MM treatment consists of induction therapy, with or without autologous stem cell transplantation (ASCT), for younger patients, with additional lines of therapy for progressive and/or refractory illness [4].
Pomalidomide is an orally administered IMiD, a structural analogue of thalidomide and lenalidomide, which exhibits enhanced efficacy and reduced toxicity [5]. The combination of pomalidomide and low-dose dexamethasone was found to demonstrate significantly longer survival and a greater number of clinical responses than DEX alone in a pivotal phase III (MM-003) trial. It was hence approved in 2013 by both the U.S. Food and Drug Administration and the European Medicines Agency for the treatment of patients with relapsed and refractory MM, who have received at least 2 prior treatment regimens, including both lenalidomide and bortezomib [6, 7].
Pomalidomide exhibits antimyeloma properties and exerts both tumouricidal and immune-stimulating effects. It is believed to bind to cereblon, a constituent of the E3-ubiquitin ligase complex, which is the principal molecular target of all IMiDs. Binding results in the ubiquitination and subsequent proteasomal degradation of the transcription factors Ikaros and Aiolos, both of which play crucial roles in the development of B and T-cells [8, 9]. Numerous immunomodulatory mechanisms have been identified, one of which is the downregulation of proinflammatory cytokines such as tumour necrosis factor α, interleukin 12 (IL-12), and IL-6 [10, 11]. Additionally, natural killer cell cytotoxicity can be augmented directly and by stimulation of T-cells via heightened levels of IL-2 and interferon-γ [12, 13]. In addition, the suppression of angiogenesis by reducing the levels of vascular endothelial growth factor, inhibiting the cell cycle, and stimulating apoptosis is also observed. The treatment has been validated through clinical trials conducted on patients who were unresponsive to lenalidomide [14].
Effective personalized cancer therapy requires the identification of biomarkers that can predict treatment response. This is particularly pertinent for MM patients, whose treatment options are expanding rapidly. The inflammatory response is a significant element in the onset and progression of several malignancies [15]. In the development of MM, cytokines and chemokines facilitate intricate interactions between malignant plasma cells and the milieu of the bone marrow [16]. The past years have seen growing interest in the tumour microenvironment, particularly the role of its constituent inflammatory cells and mediators. The tumour microenvironment comprises several key elements, including peripheral leukocytes, neutrophils, lymphocytes, platelets, and acute-phase proteins, which contribute to the inflammatory response and can be conveniently detected. A novel biomarker proposed by Hu et al. [17], viz. the systemic inflammation index (SII), measures the immune and inflammatory status of the entire body by multiplying the neutrophil count (N) by the platelet count (P) and dividing it by the lymphocyte (L) count. An elevated SSI is associated with higher incidence [18] and poorer prognosis in certain solid tumours [19]. In addition, even a lowered SSI may indicate a poorer prognosis in certain types of lymphoma, such as primary central nervous system lymphoma [20].
The aim of the study was to assess the clinical value of SII in a homogeneous cohort of MM patients treated with a pomalidomide and dexamethasone (Pd) regimen.
Material and methods
Study design
This retrospective study included all patients who received a Pd regimen according to the Ministry of Health’s drug reimbursement program for MM patients (B.54) between November 2018 and July 2022 at the Department of Hematology, Medical University of Łódź, Poland.
Patients and methods
The study cohort included 54 patients, and the SII was evaluated before the first dose of Pd. Inclusion and exclusion criteria, drug dosing, and monitoring were set according to the reimbursement program. The enrolled patients fulfilled the following criteria: At least 2 lines of treatment had been previously administered (including lenalidomide and proteasome inhibitor-containing therapies), disease progression had occurred during the last treatment, an absolute neutrophil count of ≥ 1 × 109/l, and a platelet count of ≥ 50 × 109/l (lower values were acceptable if attributable to disease activity).
Each recommended treatment cycle lasted 28 days and included the following procedures: Pomalidomide 4 mg daily on days 1 to 21, and 40 mg dexamethasone orally once a day on days 1, 8, 15, and 22 (20 mg in patients above 75 years old). Adverse events were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. [21]. Response to treatment and relapse/progression events were classified according to the International Myeloma Working Group (IMWG) [22]. The study was conducted according to good clinical and laboratory practice and the principles of the Declaration of Helsinki. The study was approved by the local ethical committee (Ethical Committee of the Medical University of Łódź, No. RNN/103/16/KE).
Systemic inflammation index evaluation
The systemic inflammation index was calculated from peripheral blood counts of platelets, neutrophils, and lymphocytes collected shortly before commencement of Pd treatment using the equation: SII = N × P/L, where N, P and L are the counts per litre of peripheral blood for neutrophils, platelets, and lymphocytes, respectively. The median time from diagnosis of MM progression (during previous therapy) to the pomalidomide-dexamethasone administration was 7 days (interquartile range, IQR: 3–21 days). None of the patients received granulocyte colony-stimulating factor (G-CSF) in the month before pomalidomide commencement and complete blood count data collection.
Statistical analysis
The distribution of continuous variables was first determined using the Shapiro-Wilk test. The mean and standard deviation (SD) were reported for normally distributed variables, while the median and IQR were used for non-normally distributed variables. The impact of the variables on progression-free survival (PFS) and overall survival (OS) was assessed using univariate and multivariate Cox’s proportional hazards models and log-rank tests. Cut-off Finder was used to establish an optimal cut-off for SII [23]. All statistical analyses were performed using Statistica 13.1 (TIBCO, Palo Alto, CA, USA), and p-values less than 0.05 were considered significant.
Results
Study group characteristics
The demographic, clinical, and laboratory characteristics of the MM patients enrolled in the study are presented in Table 1. The study group comprised 25 men (46.3%) and 29 women (53.7%). The median age at diagnosis was 63.3 years (IQR: 55.4–69.3). According to the International Staging System (ISS), 14 (25.9%) patients were classified as ISS 1, 18 (33.3%) as ISS 2, and 22 (40.7%) as ISS 3. Bortezomib, cyclophosphamide, and dexamethasone was the most prevalent first-line treatment regimen in our cohort (19; 35.2%), followed by cyclophosphamide, thalidomide, and dexamethasone (14; 25.9%). Most patients (28; 51.9%) had undergone ASCT or (27; 50.0%) radiotherapy before Pd administration. The median age at Pd administration was 63.3 years (IQR: 61.7–72.3); 38 (70.4%) patients had bone disease, 22 (40.7%) Hb < 10 g/dl, 4 (7.4%) creatinine > 2 mg/dl, and none had hypercalcaemia. Pomalidomide and dexamethasone constituted the primary third (21; 38.9%), fourth (20; 37%), fifth (12; 22.2%), and sixth treatment lines (1; 1.9%). Cytogenetic data were available for 16 (29.5%) patients; 11 (20.4%) were placed in a high-risk group according to IMWG, whereas 5 (9.26%) were in the standard-risk group.
Table 1
The characteristics of the multiple myeloma patients included in the analysis
[i] ASCT – autologous stem cell transplantation, CR – complete response, CTD – cyclophosphamide, thalidomide, dexamethasone, Hb – haemoglobin, IMWG – International Myeloma Working Group, ISS – International Staging System, LCD – light chain disease, PD – progressive disease, PR – partial response, SD – standard deviation, VCD – bortezomib, cyclophosphamide, dexamethasone, VGPR – very good partial response
Adverse events
During the treatment, 11 patients (20.4%) had a grade ≥ 3 infection, 21 (38.9%) grade ≥ 3 neutropaenia, 13 (24.1%) grade ≥ 3 anaemia, 6 (11.1%) grade ≥ 3 thrombocytopaenia, and 2 (3.7%) nephrotoxicity. Dose reduction had to be applied in 10 patients due to side effects, mainly neutropaenia (n = 5), followed by polyneuropathy (n = 3) and anaemia (n = 2). In addition, individual cases of infection (n = 1), a tendency to myelosuppression (n = 1), and unspecific neurologic symptoms (n = 1) were noted. Despite dose reduction, one patient with polyneuropathy developed neutropaenia, which required a further dose reduction.
Treatment outcomes
The median number of completed treatment cycles was 5 (IQR: 1–12). Following the third treatment cycle, one patient (1.9%) achieved complete response (CR), 2 patients (3.7%) achieved very good partial response (VGPR), 10 patients (18.5%) achieved partial response (PR), 21 patients (31.9%) had SD, and 19 patients (35.2%) had progressive disease. After the sixth treatment cycle, one patient achieved CR, 7 patients (13%) achieved VGPR, 9 patients (14.8%) achieved PR, 15 patients (31.9%) had SD, and 19 patients (35.2%) had progressive disease. It is noteworthy that 4 patients (7.14%) did not complete the first treatment cycle. The median OS and PFS in the cohort was 14.8 and 6.8 months, respectively.
Univariate analysis found that ISS 3 (HR = 2.3, 95% CI: 1.2–4.6, p = 0.01) and bone disease during Pd administration (HR = 2.9, 95% CI: 1.1–7.7, p = 0.03) negatively affected OS. In addition, Hb < 10 mg/dl at administration (HR = 1.9, 95% CI: 1.0–3.6, p = 0.04) was related to shorter PFS but not to CR/VGPR response (HR = 0.2, 95% CI: 0.1–0.6, p < 0.01). Only SII (continuous variable) affected both OS (HR = 1.0014, 95% CI: 1.00003–1.0027, p < 0.05) and PFS (HR = 1.0014, 95% CI: 1.0001–1.0027, p = 0.03) negatively. More detailed results are presented in Table 2.
Table 2
Univariate Cox hazard analysis for overall survival and progression-free survival
The variables found to be significant in the univariate analysis were included in the multivariate analysis together with established prognostic factors. At this step, SII was dichotomized to increase the clinical applicability of the results. The optimal cut-off of SII was established using Cut-off Finder and defined as the point demonstrating the most significant (log-rank test) split for OS [23]. The systemic inflammation index cut-off was estimated to be 374 and was included in the multivariate analyses. The detailed results of multivariate analyses are presented in Table 3. The systemic inflammation index > 374 (HR = 2.2, 95% CI: 1.0–4.6, p = 0.04) and bone disease at Pd administration (HR = 3.1, 95% CI: 1.2–8.0), p = 0.02) were independent predictors for OS, whereas SII > 374 (HR = 3.0, 95% CI: 1.4–6.3, p < 0.01), CR or VGPR response after any cycle (HR = 0.2, 95% CI: 0.1–0.5, p < 0.01), and Hb < 10 mg/dl (HR = 2.3, 95% CI: 1.1–4.7, p = 0.03) were independent predictors for PFS.
Table 3
Multivariate Cox hazard analysis for overall survival and progression-free survival
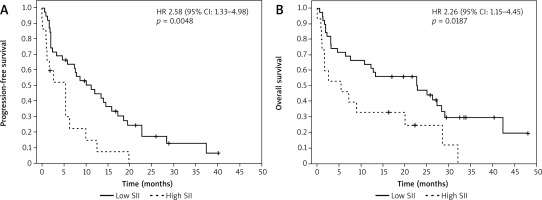
The cohort was hence split into an SII high group (SII > 374) and SII low group (SII < 374) to allow comparison of OS and PFS between them. The respective median PFS and median OS were 9.6 and 21.7 months in the SII low group, and 2.6 (log-rank p = 0.018) and 5.5 months (log-rank p = 0.035) in the SII high group. The corresponding Kaplan-Meier plots are presented in Figure 1.
Fig. 1
Kaplan-Meier plots for multiple myeloma patients treated with a pomalidomide-dexamethasone regimen according to the systemic inflammation index (SII). Patients were dichotomized at an SII value of 374. That point was selected using Cut-off Finder software. Progression-free survival (A), overall survival (B)
SSI – systemic inflammation index
Discussion
This retrospective study examined the real-life results of MM patients treated with a Pd regimen. It also evaluated the prognostic importance of a novel inflammation-related biomarker, SII, in a unique, heavily pretreated population. The findings demonstrate that high SII is an independent adverse prognostic factor for both PFS and OS in MM patients treated with a Pd regimen.
Only a limited number of publications discuss the real- life results of pomalidomide treatment. An Italian working group analysed data from 121 patients with MM who received pomalidomide and low doses of dexamethasone; the Pd regimen was the most common fourth line of treatment. The median PFS and OS were 8.5 and 14 months, respectively [24]. Maciocia et al. retrospectively analysed the data of 70 patients treated with pomalidomide at 5 centres in the United Kingdom between 2013 and 2016 [25]; their data indicate that 96.5% of patients were refractory to lenalidomide, 72.9% to both lenalidomide and bortezomib, and 92.9% to the last line of treatment. Treatment consisted of 28-day cycles of pomalidomide (given daily on days 1–21) plus dexamethasone (on days 1, 8, 15, and 22), with or without a third drug. With a median follow-up of 13.2 months, median PFS was 5.2 months and median OS was 13.7 months.
Charlinski et al. described 50 patients with relapsed/refractory myeloma, who were treated with pomalidomide in combination with dexamethasone or pomalidomide in combination with dexamethasone and bortezomib [26]. The median PFS and OS were 10.0 and 14.0 months, respectively. In addition, a recent report based on real-world data from Hungary indicated a median PFS and OS of 9.0 and 16.5 months, respectively [27] The results were quite optimistic because the study group was heavily pretreated with a median of 4 prior therapy lines. However, almost all patients in this cohort received pomalidomide in combination with an alkylating agent or a proteasome inhibitor. Considering the PFS and OS times and response rates achieved in our present cohort, and assuming some discrepancies with the groups described above, it appears that our treatment efficacy data are similar to those reported in the literature.
Our present study evaluated the prognostic significance of SII, which might serve as a more impartial indicator depicting the equilibrium between the host’s inflammatory and immune responses. Gaining more profound insight into the roles of neutrophils, platelets, and lymphocytes will aid in comprehending the intricate relationships between cancer, immunity, and inflammation. Inflammation is known to promote cell proliferation and survival, and thus tumour growth [28]. It has been proposed that an aberrant immune response, directed at either self-proteins or pathogens, heightens the chances of genetic changes and consequent transformation into MM [29]. Persistent antigenic stimulation might also induce genomic instability in MM by activating cytidine deaminases, a mechanism proposed to elucidate the transition from smouldering MM to MM [30]. In MM, all of the components of SII, viz. neutrophils, platelets, and lymphocytes, are frequently affected by various causes, including cooccurring infection, infiltration of the bone marrow, and myelotoxic effects of administered therapies.
Neutrophils both promote and inhibit the initiation, growth, and metastasis of cancer, and these contrasting functions are associated with the existence of distinct neutrophil subpopulations [31]. Normal neutrophils possess antimicrobial and anticancer properties; therefore, a functional transformation or abnormal cell differentiation must occur during carcinogenesis. There is a theory that the microenvironment associated with malignancy may trigger the reprogramming of neutrophils [31]. Numerous studies highlight the prognostic significance of several indices based on complete blood count, including neutrophil count [32, 33]. Neutrophils in peripheral blood from MM patients have been found to demonstrate various functional differences, including up-regulation of CD64 (inducible Fc gamma RI) and down-regulation of CD16 (constitutive Fc gamma RIIIa), reduced phagocytic activity, and oxidative burst; taken together, these factors contribute to increased immune suppression [34]. Thus, neutrophils might play a role in MM by aiding susceptibility to infections and contributing to the immunological dysfunction that drives tumour progression.
Cancer-infiltrating lymphocytes can inhibit growth and metastasis [35]. Thus, decreased lymphocyte count may indicate a weakening of immunological response, which may be additionally impaired by the large amount of reactive oxygen species released by neutrophils [36]. Multiple myeloma secretes thrombopoietin and IL-6, which may contribute to increased platelet count. Platelets can increase cancer progression by inducing angiogenesis, sustaining proliferative signals, and decreasing pro-apoptotic signals [37]. Epithelial-mesenchymal transition (EMT), a process involved in the metastasis of solid tumours, is known to be initiated by platelets; however, EMT is not restricted to epithelial tumours and occurs in other types, as well as in MM [38]. Thus, an increased count of platelets is potentially associated with a higher risk of metastasis, which is believed to be driven by the attachment of tumour cells to platelets through tissue factor and/or L- and P-selectins to form microemboli or microthrombi, and their further transport to target organs [39].
In addition to its proven prognostic significance in solid tumours, several studies indicate that high SII was related to shorter PFS and OS in Hodgkin lymphoma and diffuse large B-cell lymphoma [40, 41]. In addition, SII demonstrates a U-shaped relationship with the risk of death, where substantial decreases and increases of SII were associated with poorer OS for primary central nervous system lymphoma [20]. Our findings are the first to demonstrate the prognostic significance of SII in MM patients.
In our study, we have established the optimal SII cut-off at 374. This value is comparable to 330, which was provided by Hu et al. in the SII pivotal study [17]. In one meta-analysis of 15 studies, including 4577 patients with solid tumours, the median cut-off for high SII was 572 (range = 300–1600) [42]. In haemato-oncological malignancies, where a reduction in platelet count is often observed in the active disease, which is in the numerator of the SII formula, slightly lower cut-off values are expected.
As we have shown, patients with a high SII may derive less benefit from the Pd regimen. Considering this, we can hypothesize that these patients should be eligible for novel therapies. Recently, 2 novel pomalidomide-based triplets were approved for the treatment of relapsed and refractory MM with ≥ 2 prior lines of treatment, and they are reimbursed in Poland. Pomalidomide-based triplet with a novel agent, elotuzumab-anti-SLAMF7 monoclonal antibody, was evaluated in the phase III ELOQUENT-3 trial, demonstrating a significant improvement in terms of PFS (median PFS 10.2 vs. 4.7 months) and OS (median OS 29.8 vs. 17.4 months) over Pd [43]. The second pomalidomide-based triplet, isatuximab-Pd (Isa-Pd) was recently approved based on the results of the phase III ICARIA trial, in which 307 relapsed/refractory MM patients were randomized to receive the triplet or Pd. After a median of 52.4 months of follow-up, Isa-Pd demonstrated a median OS of 24.6 versus 17.7 months for the doublet [44]. Besides the novel pomalidomide-based triplets, novel immunotherapies were recently approved for relapsed and refractory MM, including CAR-T therapies-idecabtagene vicleucel and ciltacabtagene autoleucel, and bispecific T-cell engagers: teclistamab-cqyv and talquetamab-tgvs; however, these treatment options at the time of writing this article are not reimbursed in Poland.
A major limitation of our study is the absence of cytogenetic data. In Poland, the availability of cytogenetic diagnostics for MM has been limited in recent years; even if this data were present, cytogenetics would have a minimal influence on therapeutic decisions in routine clinical practice due to the lack of new drugs. Until recently, outside of clinical trials, only younger patients underwent cytogenetic analysis as part of the standard/dual ASCT qualification procedure. Only the recent reimbursement of novel medications in Poland, such as daratumumab (2019), carfilzomib (2019), and ixazomib (2021), has resulted in the implementation of routine cytogenetics testing. Nevertheless, despite the scarcity of cytogenetic data, the present study included identified high-risk cytogenetics as a component in the multivariate model in addition to ISS stage – another well-established MM outcome predictor. We hypothesize that incorporating cytogenetics data may enhance the clinical value of our observations, but we are unable to test this theory due to the absence of bone marrow samples for FISH/cytogenetics testing. Nonetheless, we were able to demonstrate that SII has a statistically significant influence on PFS and OS.








