Introduction
Clostridioides difficile is responsible for a significant number of infections in developed countries [1]. Over the years, the incidence of C. difficile infection (CDI) has increased significantly [2]. Polish researchers found that the annual CDI index was 6.1, 8.6, and 9.6 per 10,000 patient-days in 2011, 2012, and 2013, respectively [3]. During hospitalisation, the risk of developing CDI increases sixfold [4]. The classic risk factors for CDI include conditions involving immunodeficiency, antibiotic therapy, advanced age, use of drugs that inhibit gastric acid secretion, stays in intensive care units, and prolonged hospitalisation [5]. Metronidazole and vancomycin, commonly used in the treatment of CDI, may also increase the risk of CDI [6, 7]. Patients with inflammatory bowel disease (IBD) have an increased risk of developing CDI, which may result in a worse prognosis, prolonged hospitalisation, or the need for pancolectomy [8–11]. IBD patients are predisposed to CDI, probably due to dysbiosis and the use of immunomodulators [5–7, 12–14].
IBD in patients with CDI occurs in younger individuals with no history of hospitalisation or antibacterial therapy in the previous few months [11, 15]. Immunosuppression used in IBD further increases the risk of developing CDI. Patients with IBD are more likely to develop complications of CDI, such as toxic megacolon and/or intestinal perforation [11]. A large meta-analysis of patients with ulcerative colitis (UC) showed that the coexistence of CDI was associated with an increase in the risk of colectomy [16]. A higher rate of CDI recurrence and a higher mortality rate in IBD patients compared to the general population has also been shown [9, 17, 18]; however, not all scientists share this opinion [8, 11]. The occurrence of CDI in patients with IBD is a significant clinical problem [19]. Diagnosing CDI in patients with IBD is of key importance in selecting the proper therapeutic management. The risk factors for CDI include the type of IBD (CDI was more common in patients with UC) and the location and extent of the disease. CDI may lead to a worsening of the underlying disease in IBD patients.
Material and methods
A total of 204 patients with IBD and coexisting CDI hospitalised in the Department of Internal Diseases and Gastroenterology of the Central Clinical Hospital of the Ministry of Interior and Administration in Warsaw during 3 years were included in the study. All were over 18 years old.
The patients were divided into 2 groups:
50 patients with IBD without coexisting CDI (IBD + CDI-): 29 patients with Crohn’s disease (CD) and 21 patients with UC,
77 patients with IBD and CDI (IBD + CDI+): 53 patients with CD and 24 patients with UC.
Before the study began, approval was obtained (80/2016) from the Bioethics Committee at the Central Clinical Hospital of the Ministry of Interior and Administration in Warsaw. A single-centre retrospective study was conducted based on an analysis of medical records. The diagnosis of CDI was performed according to the guidelines of the European Society for Clinical Microbiology and Infectious Diseases. No deaths were noted during the study. The diagnosis of IBD was based on current guidelines; no new diagnoses were made during the hospitalisation in question in any of the patients. The analysed data included basic information such as age, sex, body weight, height, and laboratory test results. Data regarding disease history included CDI risk factors, such as use of antibiotics, immunomodulators, or proton pump inhibitors (PPIs), biologic therapy, stays in a long-term care home or intensive care unit, history of surgery, HIV infection, chemotherapy, parenteral nutrition, or PEG feeding.
Statistical analysis
Comparisons between the groups regarding demographic information, clinical data, laboratory test results, and risk factors were performed using the chi-squared test or the Mann-Whitney test. To study the relationship between potential risk factors and CDI in IBD patients, we used univariate and multivariate logistic regression models and calculated the odds ratio (OR) with 95% confidence intervals (CI) for CDI in IBD. For parametric data, mean values and standard deviation (SD) were calculated. The level of statistical significance was p < 0.05. Statistical analysis was carried out using R software, version 4.0.5 (http://cran.r-project.org). Univariate and multivariate logistic regression analysis was used to identify predictors of CDI. A stepwise “backward” approach with AIC criterium was used to select the final list of predictors in the multivariate model. Evaluation of the multivariate model using the χ2 test confirmed that all variables jointly were significant (p < 0.001). The Nagelkerke coefficient R2 had a high value (75%), indicating a good quality of the model. Additional assessment with the Hosmer-Lemeshow GOF test (p = 0.338) also confirmed a good fit of the model to the data.
Results
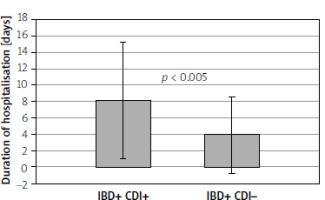
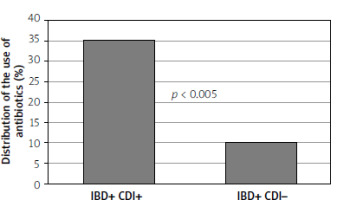
Table I presents the anthropometric data of the study groups, the history of disease, the duration of hospitalisation, the analysis of therapy, and other potential risk factors. The study showed a statistically significant relationship in terms of age between the groups of patients. Patients with IBD and CDI were significantly younger than those with IBD without CDI. It was also shown that patients with IBD and CDI had a significantly lower body mass index (BMI). Patients with IBD and CDI had significantly longer hospitalisation time than patients with IBD without CDI (Figure 1). Table I also presents the results of basic biochemical tests and blood counts of the study groups. There was no statistical significance in the results of laboratory tests among the groups of patients. Table II summarises the univariate logistic regression models for CDI. With an increase in age by 1 year, the risk of becoming infected with C. difficile was reduced by 14% (OR = 0.86, 95% CI = 0.81–0.90, p < 0.001). There was also a statistically significant correlation between the occurrence of CDI and BMI: as BMI increased by 1 kg/m2, the risk of CDI fell by 18% (OR = 0.82, 95% CI = 0.74–0.90, p < 0.001). With an increase in the concentration of albumin by 1 g/dl, the risk of CDI was reduced by 62% (OR = 0.38, 95% CI = 0.14–0.88). As the duration of hospitalisation increases, the risk of CDI tends to increase. Extending the duration of hospitalisation by 1 day increased the risk of CDI by 14% (OR = 1.14, 95% CI = 1.06–1.25). Moreover, patients receiving antibiotic therapy had a significantly higher risk of CDI than those without antibiotic therapy (OR = 4.86, 95% CI = 1.85–15.29, Figure 2). In addition, patients receiving glucocorticoid therapy had a greater risk of getting a CDI than patients without glucocorticoid therapy (OR = 3.62, 95% CI = 1.71–7.97). Patients who received azathioprine had a higher risk of developing CDI than patients without immunosuppressive treatment (OR = 3.39, 95% CI = 1.63–7.25). Patients with gastritis and/or duodenitis had a much higher risk of CDI than patients with no history of gastroduodenitis. Potential risk factors with a p-value of < 0.05 in the univariate analysis were included in the multivariate analysis. The results of the multivariate analysis are shown in Table III. The multivariate model resulted in the following combination of predictors for CDI: age, hospitalisation time, antibiotic use, and gastritis and/or duodenitis.
Table I
Baseline demographic and clinical characteristics of the study population
Table II
Univariate logistic regression models for CDI
Discussion
In the study, it was found that patients with IBD had a higher risk of CDI during antibiotic therapy compared to IBD patients who were not taking antibiotics, which confirms the theory that the use of antibiotics is a risk factor for CDI in patients with IBD. Broad-spectrum penicillins, cephalosporins, clindamycin, and fluoroquinolones entail a higher risk of developing CDI than other antibiotics [20]. The risk of developing CDI in patients using 2, 3, and 4/5 antibiotics was 2.5 (95% CI = 1.6–4.0), 3.3 (95% CI = 2.2–5.2), and 9.6 (95% CI = 6.1–15.1), respectively. This research revealed an effect of using systemic steroids on the occurrence of CDI among IBD patients. Schneeweiss et al. demonstrated that the use of systemic steroids increases the risk of CDI in patients with IBD threefold [21], but other immunomodulators were not shown to influence this risk [21, 22]. On the other hand, Issa et al. demonstrated that immunomodulators double the risk of CDI in IBD patients (OR = 2.56, 95% CI = 1.28–5.12) [22]. The current study did not confirm the effect of oral PPI use on the occurrence of CDI in patients with IBD. However, gastroduodenitis was shown to influence the development of CDI in IBD patients. This may be due to the different times of CDI detection and of PPI therapy initiation from the beginning of the first symptoms. Inflammation of the gastric and/or duodenal mucosa has the effect of changing the microbiome, thereby promoting CDI. One limitation of this study is its retrospective character. Due to the study design, the data on the use of PPIs before hospitalisation are incomplete. A common routine practice in hospitals is to discontinue the PPI on the first day of hospitalisation. In the last decade, there has been an increase in the use of PPIs. For many years, the use of PPIs has been of interest to scientists in the context of risk factors for the development of CDI [23–27]. The use of PPIs is an established risk factor for CDI as well as for recurrent CDI, which appears to be due to an effect on the microbiota, resulting in dysbiosis and colonisation of C. difficile [28–30]. The risk of CDI is present even at the initial stage of PPI therapy [25, 31, 32]. The risk of developing CDI persists for up to 12 months, even after stopping the treatment [33]. A meta-analysis of 50 studies showed an additive effect of the combined therapy with PPIs and antibiotic therapy on the development of CDI (OR = 1.97 for antibiotics, 1.82 for PPIs, and 3.44 for combined therapy) [26]. The meta-analysis confirmed the relationship between the use of PPIs and the occurrence of CDI (OR = 1.26, 95% CI = 1.12–1.39). The risk was higher among hospitalised patients than those receiving outpatient medical care [34]. Bolukcu et al. demonstrated IBD as an independent risk factor of CDI for outpatients (OR = 6.8, 95% CI = 1.5–30.08, p = 0.01) [35]. A higher CDI incidence rate has been demonstrated in outpatients with IBD compared to hospital-acquired CDI (20.2% vs. 9.7%) [36]. In the current study, there was an influence of prolonged hospitalisation on the risk of CDI in patients with IBD. A 1-day extension of hospitalisation was associated with a 14% higher risk of developing CDI. Prolonged hospitalisation is also a factor of developing CDI in IBD patients [37]. The study showed that IBD patients with CDI are statistically significantly younger than those with IBD without CDI. It has been shown that with increasing age among IBD patients, the risk of CDI decreases. Thus, an age above 65 years, which is a classic risk factor for CDI, is not connected with patients with IBD [20, 38]. According to the literature, patients with IBD and CDI are younger patients (median age = 38.5 years). and 76% of CDIs are outpatient-acquired infections [15, 35]; however, not all scientists agree [39]. In this study, lower haemoglobin concentration or thrombocytopaenia were not found to be risk factors for the development of CDI in patients with IBD. In a study by Gu et al. on a group of 260 IBD patients, it was shown that low haemoglobin concentration was a risk factor for the development of CDI [40]. In the current study, a statistically significant correlation between the occurrence of CDI and BMI was found. Hypoalbuminaemia was found to be a risk factor for CDI in IBD patients.
Conclusions
The risk factors for CDI in patients with IBD include younger age, malnutrition (lower BMI and hypoalbuminaemia), the use of thiopurines, antibiotics, or systemic steroids, prolonged hospitalisation, and gastritis and/or duodenitis. Biological therapy and PPIs were not found to be risk factors for CDI in patients with IBD.