Introduction
The most common location of intracranial aneurysms, observed in 35% of cases, is the anterior communicating artery (ACoA). Based on the macroscopic shape classification, saccular aneurysms occur in up to 90% of cases on the ACoA complex [1].
Surgical clipping was the first method used for treating ACoA intracranial aneurysms. Nowadays, endovascular treatment has become an alternative and primary method of choice. Endovascular coiling, used for the first time by Guglielmi et al. in 1990, has been used since then as the most effective technique of intracranial aneurysm embolization [2]. The technique involves insertion of detachable coils into the aneurysm sac under fluoroscopic imaging. Currently available embolization coils are made of pure platinum or platinum and hydrogel polymer [3]. Coils with a larger diameter, 0.020-inch (Penumbra 400), enable a higher packing density to be achieved in large aneurysms and thus can better protect against recanalization [4]. Further modifications of standard coiling invented to increase the efficacy of wide-necked aneurysms treatment include balloon-assisted and stent-assisted coiling. In the most complicated cases flow diverters could also be applied [5]. However, most importantly, it is clinical experience in the use of endovascular techniques that has led to a decline in the incidence of complications [2].
In the majority of patients, the first symptom of intracranial aneurysm is subarachnoid haemorrhage (SAH). The SAH is associated with a particularly high mortality rate of 32% to 67% [6]. The symptoms of functional neurological deficit depend on the location and volume of the haemorrhage. The volume of subarachnoid haemorrhage can be classified in CT scans using the Fisher grade, which enables one to predict morbidity and mortality in patients with SAH [7]. The ACoA aneurysms are at high risk of rupture due to their location related to a poor Fisher grade [8].
Since the publication of the results of the International Subarachnoid Aneurysm Trial providing evidence for the efficacy of endovascular coiling [9], there has been a constant need for gradual improvement of endovascular embolization results and safety.
Aim
The aim of our study was to assess the immediate and mid-term results of endovascular treatment of ACoA aneurysms using a wide range of methods. We performed analysis of morphological, angiographic and clinical data to estimate the factors affecting efficacy and safety of the treatment.
Material and methods
The study obtained approval from the Institutional Bioethics Committee. Subsequetly, 522 embolizations of intracranial aneurysms from the period between January 2012 and December 2016 performed in Department of Angiography and Interventional Radiology of University Hospital in Krakow were retrospectively analysed. One hundred and nine patients with ACoA aneurysm were selected for the final analysis, in which a total of 111 endovascular embolization procedures were performed. In this group, 80.7% (n = 88) of aneurysms were ruptured and 19.3% (n = 21) unruptured. In addition, 6 (5.5%) patients treated initially with surgical clipping developed recurrent aneurysms that manifested with SAH in 5 cases.
The mean age of the patients was 56.7 ±15.2 years and age ranged between 28 and 91 years. There were 50.5% (n = 55) females and 49.5% (n = 54) males. In all patients ACoA aneurysms were detected by CT angiography.
The decision regarding the choice of treatment method (surgical or endovascular) was made individually based on many factors: the anatomy of the ACoA aneurysm complex (size, dome direction, width of aneurysm neck, arteries branching from the aneurysm); the presence of atherosclerosis; Fisher and WFNS grade and the presence of parenchymal hematoma. The main factors influencing the choice of endovascular method were the aneurysm neck diameter and the origin of pericallosal arteries.
All procedures were performed under general anaesthesia using a mono-plane Axiom Artis system (Siemens Healthcare GmbH). Routinely, from the femoral access a 5 or 6 Fr guiding catheter was used. In cases of internal carotid artery kinking, a 6 Fr long sheath with a coaxial distal access catheter, 5 or 6 Fr, was applied. Continuous infusion of heparinized saline into the lumen of guiding catheters was used. As the initial part of each procedure three-dimensional digital subtraction angiography (3D DSA) and two-dimensional (2D DSA) were obtained.
In the study group, four methods of embolization were used: coiling alone, balloon-assisted coiling, stent-assisted coiling and double stenting with coiling (Y-stenting, T-stenting and X stenting). The numbers of aneurysms treated with each method of endovascular embolization can be found in Table I.
Table I
Comparison of the most important parameters of aneurysm and treatment depending on the method of embolization
During the procedures, a wide range of coil types was applied. Coils were manufactured by MicroVention Terumo Inc. (Tustin, CA, USA), Stryker Neurovascular (Kalamazoo, MI, USA), Medtronic (previously Covidien ev3; Minneapolis, MN, USA) and Penumbra Inc. (Alameda, CA, USA). In balloon-assisted coiling, balloon catheters Scepter C and XC (MicroVention Terumo Inc., Tustin, CA, USA) were used for balloon remodelling technique. In stent-assisted coiling and double stenting with coiling, stents LEO baby (Balt Extrusion, Montmorency, France) and Solitaire AB (Medtronic, previously Covidien ev3; Minneapolis, MN, USA) were deployed. In these techniques, coils were detached into an aneurysm lumen using jailing or the coil-through method.
Patients treated with stents received double antiplatelet premedication – in unruptured aneurysms consisting of 150 mg of acetylsalicylic acid and 75 mg of clopidogrel daily for five days before the procedure; in the case of ruptured aneurysms, 600 mg of clopidogrel was administered 4 h before the procedure and 300 mg of acetylsalicylic acid immediately before the operation.
In the case of intraprocedural thrombosis, abciximab in a dose of 0.25 mg/kg (half of the dose immediately in the intravenous bolus and the remaining half in continuous intravenous infusion during 24 h) was administered. If severe thromboembolism occurred, mechanical thrombectomy was performed.
Subsequently to the end of embolization, follow-up 2D and 3D DSA were performed in order to assess the result of the procedure in the Raymond-Roy occlusion classification of intracranial aneurysms. Dyna-CT was additionally obtained in cases of intraprocedural haemorrhage, or after stent implantation to assess whether it had expanded properly. After completion of the whole procedure, the patient was transferred to the post-anaesthesia care unit (if the patient had emerged from general anaesthesia) or into the intensive care unit (if barbiturate-induced coma was maintained). If stenting was deployed, dual antiplatelet treatment (150 mg of acetylsalicylic acid and 75 mg of clopidogrel daily) was applied for the next 6 months following the procedure.
Endovascular procedures were performed by two senior radiologists with 10 years of experience in neurointerventional procedures and one junior radiologist who has permission for independent treatment.
Morphological characteristics of ACoA aneurysms and cerebral vasculature were assessed on 3D DSA images. All dimensions were measured by two independent examiners and final scores were calculated as the mean of measurements taken by every examiner. In order to assess the size of an aneurysm, width of the neck, maximal height, maximal width and second width (perpendicular to maximal width) were measured. The highest measured value was classified as maximal dimension of the aneurysm. Volumes of aneurysms were calculated using the online calculator AngioCalc (www.angiocalc.com) from linear dimensions of the aneurysm, applying the mathematical formula appropriate for the shape of the aneurysm (spherical, ellipsoidal or bilobed). Packing density was also calculated using the AngioCalc calculator, as a percentage of coil volume in relation to the aneurysm volume. Medical history of patients was analysed for: the Glasgow Coma Scale (GCS), the World Federation of Neurosurgical Societies (WFNS) and the Hunt-Hess score at the moment of admission to hospital; comorbidities; duration of hospitalization; postprocedural complications (e.g. delayed ischaemic neurological deficit (DIND)) and deaths during hospitalization; aneurysm retreatment, its method and interval after the initial procedure; score on the modified Rankin Scale (mRS) 1 month after discharge and the result in the Raymond-Roy Occlusion Classification (RROC) and the interval to the follow-up examination (angiography or magnetic resonance imaging (MRI)). Good clinical outcome was defined as a score of 0 to 2 on the mRS [10].
Statistical analysis
Statistical analysis was performed using Statistica 12.5 software (StatSoft Inc., Tulsa, OK, USA). All results of the calculations were described as mean values ± standard deviation. Pearson’s correlation coefficient was calculated in order to assess the strength of correlation between two continuous variables. Pearson’s chi-square (χ²) test was used for categorical variables and the independent two-sample Student’s t-test for continuous variables. Multivariate logistic regression analysis was also performed – final models were created using the backward elimination method, with a threshold at the probability level of 0.10 for removal. Statistical significance was set at p < 0.05. The odds ratios (OR) for comparison between groups were calculated using a multivariate logistic regression model.
Results
The most important data regarding morphological and clinical parameters, treatment methods and their results were collected in Table I.
Morphological features of aneurysms
The maximal dimension and volume of the aneurysm increase proportionally to the age of the patient (r = 0.225 for maximal dimension, r = 0.237 for volume; p < 0.05). Males tend to have larger aneurysms (volume: 189.6 ±250.7 vs. 104.4 ±112.9 mm³, p = 0.024; maximal dimension: 8.4 ±4.1 vs. 6.9 ±3.0 mm, p = 0.020) with wider necks (4.0 ±1.7 vs. 3.4 ±1.4 mm, p = 0.029), in comparison to females.
The ACoA aneurysms with coexisting aplasia or a hypoplastic A1 segment had a significantly higher maximal dimension (8.8 ±3.9 vs. 7.2 ±3.4 mm, p = 0.031) and a wider neck (4.2 ±1.8 vs. 3.5 ±1.5 mm, p = 0.023) than those accompanied by a well-formed A1 segment.
Subarachnoid haemorrhage
80.7% (n = 88) of treated patients had SAH due to ACoA aneurysm rupture. In the study group, 36.4% (n =39) of patients had a GCS score equal to or less than 8 and 37.0% (n = 40) of patients had a Hunt-Hess grade of 4 or 5.
Treatment results
Immediately after the procedure 56.9% (n = 62) of patients had RROC class I, 37.6% (n = 41) class II, and 5.5% (n = 6) class III. Mean packing density of ACoA aneurysms was 27.2 ±13.0%. No differences in RROC (1.5 ±0.6 vs. 1.5 ±0.7; p = 0.933) or packing density (27.3 ±13.2% vs. 26.6 ±12.2%; p = 0.826) were found between ruptured and unruptured aneurysms. Advanced methods of embolization (balloon-assisted, stent-assisted coiling and double stenting with coiling) also had no impact on packing density in comparison to coiling alone (p = 0.322; p = 0.247; p = 0.456).
Irregular aneurysms (61.3% vs. 35.9% for regular aneurysms, p = 0.016) and those with posterior orientation of the dome (84.6% vs. 37.5% for anterior orientation; p = 0.001) were significantly more frequently incompletely occluded (RROC class II and III). Multivariate analysis for complete embolization confirmed the strong influence of above-mentioned parameters on the extent of embolization (irregular shape of aneurysm: p = 0.009, OR = 3.333; anterior/posterior orientation of the dome: p = 0.003, OR = 10.989).
Follow-up
Mean length of hospital stay was 15.5 ±22.0 days. There was a statistically significant difference in the length of hospital stay after the procedure in patients with or without SAH (17.8 ±23.8 vs. 5.7 ±3.0 days; p = 0.023). Moreover, the patients admitted with a GCS score of 8 or less (23.9 ±28.3 vs. 10.9 ±3.3 days; p = 0.007) as well as grade 4 or 5 on the Hunt-Hess scale (25.4 ±29.3 vs. 9.7 ±13.6 days; p < 0.001) were hospitalized significantly longer than those with better condition at admission. Overall, 25.7% (n = 28) of the patients died during hospitalization.
Of 69.1% (n = 56) patients who attended the follow-up appointment one month after the discharge, 80.4% (n = 45) had a good clinical outcome. The summary regarding GCS values at admission of patients with respective mRS grades is shown in Table II.
Table II
Distribution of scores in mRS at follow-up appointment one month after the discharge according to GCS value at admission
| mRS | GCS > 8 | GCS ≤ 8 |
|---|---|---|
| 0 | 32 (68.1%) | 2 (22.2%) |
| 1 | 6 (12.8%) | 2 (22.2%) |
| 2 | 3 (6.4%)a | 0 (0.0%) |
| 3 | 3 (6.4%) | 3 (33.4%) |
| 4 | 2 (4.2%)b | 1 (11.1%)c |
| 5 | 1 (2.1%)d | 1 (11.1%) |
| 6 | NDA | NDA |
Moreover, within the group of discharged patients, only 50.6% (n = 41) underwent follow-up radiological examination. In that group, 73.2% (n = 30) of aneurysms had a better or the same class in the RROC and 26.8% (n = 11) had recanalization.
In the group with recanalization, 6 patients (overall retreatment rate: 7.4%) had additional endovascular procedures. The remaining 5 patients remain under follow-up.
Male gender (72.7% vs. 36.7% for patients without recanalization; p = 0.040), superior orientation of the dome of aneurysm (81.8% vs. 43.3% for patients without recanalization; p = 0.029) and poor outcome on the mRS scale 1 month after the discharge (66.7% vs. 19.4% for patients without recanalization; p = 0.017) were associated with more frequent recanalization of ACoA aneurysm. Furthermore, patients with initially incomplete embolization of the aneurysm were more prone to have class II and III in the RROC in the follow-up radiological examination (60.0% vs. 23.8% for patients with initial RR I; p = 0.019). However, multivariate logistic regression for aneurysm recanalization showed that only poor outcome on the mRS scale 1 month after the discharge was a predictor of aneurysm recurrence (p = 0.045, OR = 10.417). Low packing density of the aneurysm was not associated with more frequent aneurysm recanalization, as in the group with worsening class in the RROC it was 21.1 ±7.9%, while in the group with stable or improved outcomes it was 26.3 ±12.9% (p = 0.221).
Complications
The overall intraprocedural complication rate in our study was 6.6% (n = 8), taking into account initial procedures as well as recanalization treatment procedures (n = 121). Three (2.5%) procedures were complicated by bleeding from the aneurysm, one (0.8%) by coil prolapse into the lumen of the vessel and 3 (2.5%) by formation of thrombus. Moreover, 1 (0.8%) patient suffered from intraprocedural aneurysm rupture, relocation of coils and thrombosis.
Bleeding from the aneurysms occurred in all three cases during the coil introduction and occurred significantly more often during the procedures ending with higher packing density (43.0 ±16.2% vs. 26.6 ±12.5% without complications; p = 0.012). Patients affected by the intraprocedural relocation of coils had elevated packing density as well (49.7 ±7.2% vs. 26.8 ±12.7% without complications; p = 0.012).
Intraprocedural thrombosis occurred during three procedures with stent implantation and one procedure performed in the technique of balloon-assisted coiling. Haemorrhagic complications happened to every operator, while all thrombotic events happened only to the junior radiologist.
Discussion
Intracranial aneurysms originating from ACoA have always been regarded as a challenge by neurosurgeons, taking into consideration their deep midline location and the abundance of largely varied surrounding vessels [11]. Rapid development of endovascular coiling and its supporting techniques has led to its domination over the surgical approach, and currently it is considered as a highly effective and safe method of ACoA aneurysm treatment [2, 12]. Nevertheless, constant analysis of treatment results is vital to provide high standards of ACoA aneurysm therapy.
In Table III [12–22], we compared studies which focus on the analysis of the ACoA aneurysm treatment results. We conducted a comprehensive search of the MEDLINE database between July and August 2017 (last search: 30 August 2017) using such keywords as ‘anterior communicating artery aneurysm’ and ‘endovascular embolization’ or ‘endovascular treatment’ or ‘coiling’. Preference was given to the articles published within the past 10 years in the English language. The inclusion criteria were the presence at least two out of three parameters: detailed results regarding immediate embolization results, intraprocedural complication rate and follow-up results of anterior communicating artery aneurysm embolization. Exclusion criteria: articles concentrating only on a special subtype of these aneurysms (e.g. wide-necked) or lack of detailed numerical data concerning analysed parameters.
Table III
Comparison of immediate embolization results of ACoA aneurysms from last 10 years
In the present study, we achieved the result of complete aneurysm obliteration in 56.9% of treated patients immediately after the procedure, which was a good result in comparison to other listed studies. Only four of them reached higher efficacy in terms of total occlusion, but three of these studies were conducted on notably smaller groups [12, 14, 18] and the remaining one focused mainly on unruptured aneurysms [20]. Furthermore, in contrast to the rate of immediate complete and near-complete occlusion of ACoA aneurysms reported by Fang et al. in the meta-analysis (88%), our results (94.5%) still remain favourable [23]. The vast range of supporting techniques (balloon assistance, single stent implantation, double stenting) and devices utilized during procedures (coils and stents of various manufacturers) enabled the management process to be tailored to the needs of a particular patient.
Previous literature has reported numerous morphological factors as predictors of unsatisfactory immediate embolization results, namely: ruptured aneurysmal sac, irregular shape, superior or posterior dome orientation, wide neck and large dimensions of the aneurysm [15, 24, 25]. Our results did not confirm the thesis that aneurysm rupture status influences the immediate success rate of embolization. Nonetheless, irregular shape of the aneurysm was associated with frequent incomplete embolization of ACoA aneurysms, since precise and regular deployment of coils in such an unpredictable space was in many cases almost impossible. Moreover, posterior dome orientation of ACoA aneurysm was discovered as the strongest predictor of unfavourable embolization outcome, which elevates the risk of incomplete embolization more than ninefold [13, 25].
Apart from the RROC, coil packing density is regarded in the literature as an objective method of embolization quality assessment [26]. The minimal level of packing density which diminishes the risk of aneurysm recanalization is 24% [27]. Nevertheless, that threshold was established based on two-dimensional DSA images, and its equivalent for three-dimensional technique is slightly lower – between 15.5% and 19.4% [28]. In the present study, mean packing density at the level of 27% was obtained, which is similar to mean values in other studies [13, 28]. Our investigation did not reveal any factors influencing the packing density immediately after the procedure. Even supporting techniques used during embolization, which theoretically should improve the process of dense packing, did not make any difference in the current study, contrary to the results of Kim et al. [29].
The percentage of complications in our study is within the range of values presented in other studies conducted on ACoA aneurysms, shown in Table IV [12–22]. However, our study presents quite a different distribution of adverse events, yet all three types of complication had almost equal incidence. Other manuscripts, on the other hand, reported intraprocedural rupture of aneurysm as the dominating one. The incidence of this complication was frequently linked with overpacking of the aneurysm [14, 30], which is in accordance with our results. Generally, the most common reasons for the intraprocedural rupture mentioned in the literature are: coil oversizing, aneurysm overpacking, excessively stiff coils or incautious manoeuvres of the microcatheter or even the guidewire itself [30]. We achieved a relatively low rate of this complication, which may result from the large variety of coil types used during procedures – bare platinum, hydrogel-coated, helical and 3D-shaped coils, which were selected individually. Our results confirmed that high value of packing density was a predisposing factor of coil prolapse as well. Nevertheless, it is a rather harmless complication, since minor coil displacements into the vessel lumen, without any evidence of flow disruption or thrombosis, could even be treated conservatively using anti-platelet and anti-coagulant agents [14].
Table IV
Comparison of the rate of intraprocedural complications reported in studies conducted on ACoA aneurysms from last 10 years
In our study, long-term hospitalization correlated with poor GCS score, high Hunt-Hess grade and intraprocedural complications. In the group characterized by good pre-procedural condition or without any adverse events during the procedure, length of stay was similar to that reported by Tykocki et al. [31]. Moreover, the mortality rate in our study (25.7%) seems to be relatively high, as in the majority of studies it ranges between 1% and 6.5% [14, 16, 19, 21, 22]. On the other hand, it is worth noting that the lion’s share of deaths during the hospitalization were linked with high Hunt-Hess grade at admission. Schuette et al. reported the mortality rate of 66% in the group with Hunt-Hess 5 and 32% with Hunt-Hess 4 [11]. That high level of mortality may be a consequence of the mass effect and further brain oedema resulting from severe SAH.
The percentage of patients who undergone follow-up examination was rather small in the current study, due to the low compliance and wide geographic distribution of our patients. The comparison of follow-up results of ACoA aneurysms, understood as progression, stabilization or regression in RROC, is shown in Figure 1 [12–22]. However, the mean length of follow-up is highly varied among mentioned studies, ranging from 6 months [15, 16] to 36 months [19]; thus their results are hard to compare. Previous studies also identified some factors related to the elevated probability of aneurysm recanalization, such as previous rupture of aneurysm, large size of the dome, wide neck, initial incomplete occlusion, posterior or superior dome orientation, low packing density, male gender and stent-assisted coiling [22, 26, 29, 32]. Multivariate analysis revealed that only poor mRS scale 1 month after discharge was related to the decline in the extent of aneurysm occlusion, while univariate analysis also identified male gender, initial insufficient embolization and superior direction of the dome of ACoA aneurysm as predictors of aneurysm recurrence.
Figure 1
The comparison of follow-up examination results defined as a change in Raymond- Roy occlusion class from studies on ACoA aneurysms conducted over the course of last 10 years
RR – Raymond-Roy occlusion classification, NDA – no data available.
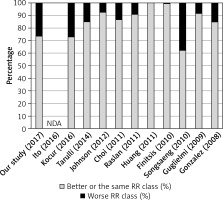
Our study identified some factors influencing morphology of the aneurysm. The most crucial factor influencing the size of the ACoA aneurysm is asymmetric distribution of haemodynamic forces and further extremely high wall shear stress concentrating in the point of aneurysm origin at the ACoA. Asymmetries in blood flow in the ACoA territory are associated with anatomical variants frequently occurring there, such as the A1 segment of anterior cerebral artery aplasia/hypoplasia [33]. In our study, concomitance of ACoA aneurysm with aplasia or hypoplasia of the A1 segment resulted in increased aneurysm dimensions and width of the neck, which is in accordance with other authors [15, 33].
In the present study, there was uneven distribution of ruptured and unruptured aneurysms between particular methods of treatment, and those which involved the use of stents were considerably more often utilized in patients without SAH. This tendency is related to the fact that the procedure of stent implantation must be preceded with antiplatelet premedication and then requires at least 6 months of its continuation in order to prevent in-stent thrombosis. It is generally known that this kind of therapy increases the risk of death in the case of re-bleeding of the aneurysm, and this is the reason why stent-assisted coiling is avoided in patients with acute SAH in spite of the proven acceptable safety profile [34]. However, three patients from our cohort were treated with stents immediately after the aneurysm rupture and all of them survived hospitalization without any complications. Thus, stent-assisted coiling should be considered in patients with ruptured aneurysm of very challenging morphology (e.g. wide-necked or giant) in very good pre-procedural condition. The stent presence is undoubtedly beneficial for preventing aneurysm recurrence, not only because of promoting thrombosis progression in the aneurysm lumen, but also by providing the scaffold for endothelialisation of the aneurysm neck [35]. Moreover, an additional advantage of the antiplatelet therapy in the postprocedural period in patients with SAH is reduction of the risk of delayed cerebral ischaemia [36].
Endovascular embolization of intracranial aneurysms is a dynamically developing branch of interventional neuroradiology and constant improvements in the technique are essential. Sometimes endovascular coiling of ACoA aneurysm is not possible or repeatedly unsuccessful and a novel approach may be beneficial. Thus far, flow diverters are mostly avoided in the ACoA territory by the majority of centres, including ours, owing to the fact that they may occlude perforating arteries arising from the ACoA, such as the recurrent artery of Heubner or medial lenticulostriate arteries, leading to basal ganglia infarction [5]. Nevertheless, some authors made an attempt of ACoA aneurysm embolization using flow diverters [5, 37]. Colby et al. in their study described a high rate of ACoA aneurysm occlusion and simultaneously low complication rate after using the flow diversion method with the Pipeline Embolization Device. The overall ischaemic complication rate in this study was comparable to the general population of patients treated with this method and other techniques of embolization [5]. In consequence, the use of flow diverters may be a good idea in daunting cases. Furthermore, the WEB Aneurysm Embolization System may be a future method of wide-necked bifurcation aneurysm treatment. This device is placed in the aneurysm lumen, where it modifies the blood flow at the level of the neck and induces intrasaccular thrombosis. The study by Pierot et al. demonstrated good efficacy and high safety of treatment with this device, and the study group also included ACoA aneurysms. However, this device requires a prophylactic antiplatelet regimen at least in the pre-procedural period, as it significantly reduces the risk of intraprocedural thromboembolic events [38]. In consequence, the use of this method in treatment of ruptured aneurysms should be implemented with additional safety precautions.
Conclusions
Endovascular embolization of ACoA aneurysms using multiple techniques (coiling alone, balloon-assisted coiling, stent-assisted coiling) has very good immediate and mid-term efficacy of the occlusion, as well as low complication rates. The most powerful factor influencing the incidence of complications is packing density, which should be maintained slightly above the level of effective occlusion. Poor mRS outcome, male gender, superior orientation of the dome and initial incomplete embolization are factors predisposing to ACoA aneurysm recurrence.








