INTRODUCTION
COVID-19 not only induces immunosuppression and cytokine storms but also lymphopaenia, impaired T and B cell immunity, and destructive tissue inflammation [1]. The pandemic caused by SARS-CoV-2 in the fourth quarter of 2019 has affected the entire world, resulting in deaths and socioeconomic problems. Healthcare workers (HCWs) have been particularly impacted by the disease. On 14 January 2021, the immunisation against SARS-CoV-2 was initiated with an inactive vaccine for HCWs in Türkiye. After vaccinating the priority risk groups [2], the general population was vaccinated with CoronaVac (Sinovac, China), an inactivated SARS-CoV-2 vaccine. Phase 1 and 2 studies have shown that it is well-tolerated and elicits an antibody response [3]. In phase 3 trials in Türkiye, the effectiveness of CoronaVac was reported to be 83.5% [4].
Although 17 approved drugs including Paxlovid® (nirmatrelvir/ritonavir) and Lagevrio® (molnupiravir) and 49 drugs under development are available [5], the most important potential agents for disease prevention are vaccinations [6]. It is expected that the exposure to SARS-CoV-2 differs depend on the working divisions. Even in the absence of laboratory and clinically detected disease, ongoing immune system stimulation may influence an individual’s antibody response to a vaccine, depending on exposure intensity.
HCWs were the first to receive the vaccination, followed by other high-risk groups. In this schedule, 2 doses of CoronaVac 600 U/0.5 ml (Sinovac Life Science Co., Ltd., Beijing, China) were given at a 28-day interval.In our study, we analysed the relevance between the professions of HCWs working in different divisions and the antibody levels they developed against the vaccine.
AIM
The aim of the study is to investigate the possible effect of exposure to varying degrees of virus density in HCWs during the pandemic period, depending on the working environment and profession, on the antibody levels that will develop after inactivated SARS-CoV-2 vaccine. Although this exposure does not cause any detected and known COVID-19 disease, it can stimulate the immune system, leading to varying degrees of immunity.
MATERIAL AND METHODS
This study was carried out retrospectively by University of Health Sciences, Bursa Training and Research Hospital, Divisions of Immunology Allergy, Internal Diseases, and Infectious Diseases Clinics between June and September 2021.
The study included 177 individuals aged between 18 and 55 years who had received 2 doses of the inactive SARS-CoV-2 vaccine, tested negative for PCR, had no known history of coronavirus, had no acquired or primary immunodeficiency, did not use immunosuppressive drugs, and tested for SARS-CoV-2 antibodies between 7 and 14 days after the second dose of the vaccine. The control group consisted of 18 unvaccinated individuals with the same characteristics.
Those with malignancy, immunosuppression, pregnancy, and a positive SARS-CoV-2 PCR test, as well as those with probable or suspected COVID-19 disease, were excluded from the study.
Study data were obtained retrospectively by selecting appropriate hospital medical records. Neutralising antibodies against the binding region of the virus S protein (RBD) were quantified. For this, Advia Centaur SARS-CoV-2 IgG (Siemens, USA) kits were used. The sensitivity of the kit was 96.4%, the specificity was 99.9%, and the reference range was 0.5–1.00 Index. The cut-off points were < 1.0 non-reactive, ≥ 1.0 reactive. Values above this were considered positive, and between 1–10 were considered effective in preventing the disease. If the antibody level was < 1, it was considered that there was no antibody response, between 1 and 10 it was considered a low-level antibody response, and > 10 it was considered a high-level antibody response. Regretfully, we were unable to locate the numbers of real values exceeding 10 within our database. Nonetheless, reference values ranging from 1 to 10 are regarded as positive for an antibody response to the vaccine, depending on the kit utilised.
The Turkish Ministry of Health as well as the Ethics Committee of the University of Health Sciences, Bursa Training and Research Hospital, approved this study with a decision dated 5 May 2021 with the number 2011-KAEK-25 2021/05-02. This study is in accordance with the provisions of the 1995 Helsinki and Edinburgh 2000 notifications.
STATISTICAL ANALYSIS
The statistical analysis program used in our study was NCSS (Number Cruncher Statistical System). Study data were evaluated using descriptive statistical methods (mean, standard deviation, median, frequency, ratio, minimum, maximum). The Fisher-Freeman-Halton Exact test was used to compare qualitative data. It was considered significant at p < 0.05.
RESULTS
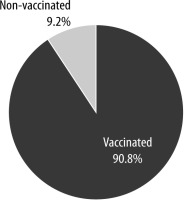
Our study included 177 people who received 2 doses of inactivated SARS-CoV-2 vaccine and 18 people who had never been vaccinated, i.e. a total of 195 HCWs. Their mean age was 39.4 ±9.1 years, 29% were male (n = 56), and 71% (n = 139) were female. The distribution of descriptive characteristics and vaccination state is shown in Table 1 and Figure 1.
TABLE 1
Distributions of descriptive features in the group of health care workers
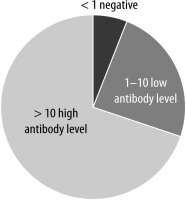
When the SARS-CoV-2 antibody test results of the participants were examined; 6.2% (n = 12) of antibody levels were negative, and 24.1% (n = 45) were positive with a low antibody response between 1 and 10, and 69.7% (n = 136) were positive with a high antibody response. The distribution of SARS-CoV-2 antibody results is shown in Figure 2.
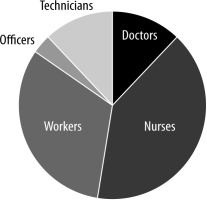
When the professions of the HCW participants were examined; 12.3% (n = 24) were doctors, 40% (n = 78) were nurses, 32.3% (n = 63) were workers, 3.6% (n = 7) were officers, and 11.8% (n = 23) were technicians. The distribution of occupations is shown in Figure 3.
The SARS-CoV-2 IgG negativity rate was 2/177 (1%) and the seroconversion rate was 175/177 (99%) in vaccinated HCWs. Of these, 45/177 (25%) had low antibody levels and 130/177 (74%) had high antibody levels. In 18 participants who were not vaccinated, who had no known or suspected history of SARS-CoV-2 infection, and whose PCR tests were also negative, the levels of antibodies were as follows: 10/18 (56%) negative, 2/18 (11%; 1 worker and 1 officer) low antibody positive, and 6/18 (33%; 3 doctors, 2 nurses, 1 worker) high antibody positive. It is important to remember that the PCR test may not have identified the mild or asymptomatic COVID-19 illness in these 6 individuals. A comparison of SARS-CoV-2 antibody results by vaccination status of participants is shown in Table 2.
TABLE 2
Comparison of SARS-CoV-2 antibody results by vaccination status of participants
According to the antibody test results, statistically significant difference was found between the vaccination position of the participants (p = 0.001; p < 0.01).
The rate of SARS-CoV-2 antibody negativity was higher in those who had never been vaccinated. A comparison of SARS-CoV-2 antibody results of vaccinated participants by occupation is shown in Table 3.
TABLE 3
Comparison of SARS-CoV-2 antibody results of vaccinated participants by occupation
There was no statistically significant difference between SARS-CoV-2 antibody results according to the occupation of the vaccinated participants (p > 0.05). A comparison of SARS-CoV-2 antibody results of vaccinated healthcare professionals by occupations is shown in Table 4. No statistically significant difference was seen between vaccinated HCWs according to SARS-CoV-2 antibody results (p > 0.05).
TABLE 4
Comparison of SARS-CoV-2 antibody results of vaccinated healthcare professionals by occupation
There was no known reported evidence of any side effects and any emergency room or outpatient clinic visits due to the COVID-19 vaccines and/or SARS-CoV-2 infection in the hospital records following vaccination during the study period. According to our clinical records, no healthcare professional applied to the allergy/immunology clinic because of local or systemic adverse reactions of vaccinations.
DISCUSSION
HCWs were a priority risk group for vaccination during the pandemic because they were the most frequent group that was not protected against SARS-CoV-2 infection. The magnitude of the risk exposed is undoubtedly directly proportional to the divisions studied. Immune responses, both cellular and innate humoral, interact intricately to produce vaccine protection. The antibody response is a crucial indicator of the immune response, even though it does not fully support the vaccine’s protective effects [7]. In our investigation, we aimed to assess the antibody titres produced by vaccination with SARS-CoV-2 in HCWs employed in various divisions. In HCWs who received 2 doses of the SARS-CoV-2 inactivated vaccine, there was no correlation observed between the working division and the antibody titres measured 4–6 weeks after the first dose, according to our study. The SARS-CoV-2 IgG negativity rate was found to be 2/177 (1%) and the seroconversion rate was found to be 175/177 (99%) in vaccinated HCWs.
In the study of Soysal et al., HCWs were administered 2 doses of CoronaVac at 28-day intervals. Antibody levels were measured 4 weeks after the second dose of the vaccine. Antibody titres were obtained in 50 (51%) of 103 previously infected HCWs and 142 (23%) of 627 uninfected HCWs. Anti-RBD antibody titres were found in HCWs with prior natural infection (median: 1220 AU/ml, range: 202–10328 AU/ml) than uninfected HCWs (median: 913 AU/ml, range: 2.8–15547 AU/ml, p = 0.032), who were significantly higher [8]. In contrast to their study, HCWs who did not have a history of suspected or confirmed SARS-CoV-2 infection and who did not have a positive PCR test result were assessed, taking into account the divisions in which they were employed in our study. Additionally, 5 to 6 weeks after the initial vaccination dose, SARS-CoV-2 antibody titres were assessed. In our study, the SARS-CoV-2 IgG seroconversion rate was found to be 175/177 (99%). The vaccine administered in both studies is an inactivated SARS-CoV-2 vaccine.
In the study of Akar et al. the ability of CoronaVac to produce antibodies was found to be 97.9% at least 28 days after the second vaccine. However, antibody responses were observed in only 25.3% of the participants in samples taken at least 28 days after the first vaccine [9]. In a phase 2 study of the vaccine, in some of the people who were vaccinated on days 0–28, the antibody response of volunteers aged 18–59 years was investigated only on the 28th day after the second dose, and the response was found to be 99.2%. This value was 96.5% on the 14th day, and after the second vaccination in the 0–14-day vaccination part of the phase 2 study, it increased to 97.4% on the 28th day [3, 10]. When samples were obtained 4–6 weeks after the initial dose in our study, 99% of the subjects had an antibody response, comprising 29% male (n = 56) and 71% female (n = 139), with a mean age of 39.4 ±9.1 years.
Dundar et al. investigated the antibody response of SARS-CoV-2 infected and uninfected HCWs after vaccination with 2 doses of an inactivated vaccine against COVID-19. Acquired immunogenicity was measured on days 27 and 42 after the first dose of vaccine. The sample size was 120. The overall seropositivity rate after the second vaccination was 97.5% in all individuals (n = 117); of these, 44 were seropositive after the first dose. They found that the percentage having previously had COVID-19 in seropositive individuals before the second vaccination (59.1%) was significantly higher than in seropositive individuals (10.96%) after the second vaccination (p < 0.0001) [11]. The sample size of this study was smaller than our study (120/195), and differently, people who were previously infected with SARS-CoV-2 were also included in the study. With the identical vaccination utilised, the SARS-CoV-2 IgG seroconversion rate in our investigation was determined to be 175/177 (99%).
Ghazy et al. scanned articles on the effectiveness of COVID-19 vaccines and found that 22 of 21,567 were suitable for quantitative analysis. They found that the mortality rate and likelihood of severe disease were significantly reduced in the vaccinated group compared to the unvaccinated group at 7 and 14 days after full vaccination [12]. This condition indicates that the antibody measurement times of our study were accurate, because they also reflect the formation of antibodies against the vaccine in the designated weeks.
The antibody levels of control subjects in our study were as follows: 10/18 (56%) negative, 2/18 (11%; 1 worker and 1 officer) low antibody positive, and 6/18 (33%; 3 doctors, 2 nurses, and 1 worker) high antibody positive in the 18 unvaccinated individuals who had no known or suspected history of SARS-CoV-2 infection and whose PCR tests were also negative. It is important to note that the PCR test might not have detected the mild or asymptomatic COVID-19 infection in these 6 high antibody positive individuals. It was thought that the presence of antibodies in these people may be due to a previous asymptomatic or mild SARS-CoV-2 disease as well.
Moreover, Table 2 shows a comparison of SARS-CoV-2 antibody results by vaccination status of participants (vaccinated vs. unvaccinated individuals). There was a statistically significant difference for low and high antibody titres between vaccinated vs. unvaccinated individuals.
We think that repeated viral exposures due to being worked constantly in the division stimulate the immune system and may cause the antibodies that develop to be at higher levels after vaccination. This is the reason why the SARS-CoV-2 IgG seroconversion rate in our study group was determined to be very high (99%).
Although the duration of the vaccine’s protection is of course debatable, the fact that patients with low and high antibody titres did not become infected with SARS-CoV-2 during the study period clearly indicates that at least some level of protection was achieved in these individuals.
LIMITATIONS
Our study has several limitations, the most significant of which is that it only included young adults in a particular occupational group and aged 18 to 55 years. The other limitations are the small sample size and unknown pre-vaccination antibody titres of the HCWs in our study. Therefore, it is not possible to generalise our results to people aged 55 years and over and to all socioeconomic levels of society. Although we included individuals who had never had SARS-CoV-2 and whose PCR tests were also negative, another limitation of our study is that the negative predictive values of the PCR tests were not considered. In our study, only antibody levels were evaluated, and data on cellular immunity could not be presented. For this reason, it is not even possible to present sufficient evidence on the extent to which the vaccine will protect individuals from the disease.
CONCLUSIONS
In HCWs who received 2 doses of the SARS-CoV-2 inactivated vaccine, there was no discernible relationship between the section worked and antibody titres measured 4–6 weeks after the first dose. The SARS-CoV-2 IgG negativity rate was 2/177 (1%) and the seroconversion rate was 175/177 (99%) in vaccinated HCWs. There was no statistically significant difference between SARS-CoV-2 antibody results according to the professions of HCWs.