Summary
The consequences of inappropriate valve sizing may significantly impact the clinical course after aortic valve replacement; therefore, the aim of this study was to assess the consistency in aortic valve sizing between surgical and transcatheter aortic valve replacement in patients treated for severe aortic stenosis. The study shows that there is a discrepancy between the results of the sizing process. In patients treated percutaneously the nominal sizes of prostheses are larger, and therefore patients could potentially achieve larger true internal diameter and effective orifice area. Further studies are needed to assess how the discrepancy in valve sizing might affect clinical outcomes, with a particular focus on patient prosthesis mismatch, aortic regurgitation, and prosthesis durability.
Introduction
Transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) are invasive treatment procedures for patients with severe aortic stenosis. The choice of procedure is determined by individual factors related to the patient’s anatomy, comorbidities, operative risk, and the informed consent of the patient. The current European and American guidelines are evolving into an optimal strategy tailored to individual patients taking into consideration safety, performance, and durability.
One of the crucial aspects of any invasive aortic stenosis treatment is the valve prosthesis selection with sizing adjusted to the patient’s anatomy and their individual haemodynamic needs. The sizing algorithms differ significantly between the surgical and percutaneous procedures. During SAVR it is possible to directly assess the native aortic annulus and perform a final prothesis sizing intraoperatively. The prosthesis choice for a TAVR procedure is based mainly on the preprocedural assessment of cardiac imaging results, i.e. computed tomography, 3D echocardiography, or angiography. However, both invasive treatment options could benefit from careful and comprehensive preprocedural assessment of the clinical factors and aortic annulus imaging.
It was established that the results of aortic annulus measurements by cardiac computed tomography (CCT) provide adequate dimensions, which are similar to intraoperative measurements. Interestingly though, comparable measurement results do not mean that the same prosthesis size would be chosen for SAVR and TAVR. Because of this the same patient treated with different invasive procedures for aortic stenosis could receive a different size of valve prosthesis. Clinical studies as well as registries showed that sizing may strongly impact the safety of the procedure as well as morbidity and mortality after valve replacement.
Aim
To-date no study has been undertaken to compare the sizes of the aortic valve prosthesis selected for surgical and transcatheter treatment for the same patient with severe aortic stenosis. The aim of this study was to assess the consistency in aortic valve sizing between SAVR and TAVR based on the standard prosthesis selection protocols for both methods. In addition, the correlation between postprocedural aortic valve performance measured by echocardiography and difference in size selection was assessed.
Material and methods
Patients
The study comprised 265 consecutive patients with aortic stenosis treated with SAVR between June 2012 and January 2016 in the Cardinal Stefan Wyszynski National Institute of Cardiology in Warsaw, Poland. Patients were included in the study if SAVR was preceded by CCT with a contrast agent to assess coronary artery disease (the period between CCT and SAVR was less than 183 days). We excluded from the study any patients with nondiagnostic imaging results and patients who underwent redo aortic valve surgery. Clinical and demographic data were acquired from the hospital database and descriptions of the cardiac procedures. The study protocol was approved by the Local Ethical Committee.
Surgical aortic valve replacement
All procedures were open heart surgeries performed via median sternotomy with the use of cardioplegia. Aortic annulus measurements were performed directly after resection of the aortic leaflets and removal of calcification from the native valve. Selection of the size for the prosthesis was based on fitting of a Hegar dilator and valve sizer provided by the valve manufacturer. The outer diameter of the sizer was the same size as the chosen valve prosthesis. However, the force of attempts to cross the native annulus with the sizer was not standardized, and final prosthesis selection was left to the operator’s discretion.
Cardiac computed tomography
The CCT examination was performed on all patients in the study to assess the extent of atherosclerosis of the coronary arteries and the need for additional coronary artery bypass grafting during aortic valve surgery. Imaging was performed with a dual-source scanner (Somatom Definition, Siemens, Forchheim, Germany) after administration of sublingual nitro-glycerine (0.8 mg). The resulting CTT scans were retrospectively analysed on a postprocessing station (Osirix MD, Pixmeo, Bernex, Switzerland) by an experienced cardiologist who was a member of the local TAVR team. The measurements of the native aortic annulus (dimensions, area, perimeter) were performed following the routine protocol according to the SCCT consensus [1]. Images from the systolic phase of the cardiac cycle were used for all measurements. In the case of artifacts impairing the diagnostic value of measurements, additional reconstructions were performed.
Prosthesis size selection
The most suitable prosthesis size was selected by a member of the TAVR team based on the CCT measurements of the aortic annulus ring. Valve size selection was based on the valve sizing guidelines according to the routine protocol for the TAVR qualification and using criteria described in the literature at that time. The TAVR prostheses choice was limited to the valves available at the time in the National Institute of Cardiology: Edwards Sapien XT (Edwards Lifesciences) and CoreValve (Medtronic). The medical staff who were taking part in the TAVR prosthesis selection were blinded to the size chosen by the cardiosurgeon during the SAVR.
Echocardiography
A transthoracic echocardiographic examination was performed in all patients before and after the SAVR following routine guidelines. Preprocedural echocardiography assessments consisted of aortic valve dimension, aortic valve velocity, other valve dysfunction, and left ventricular function. Post-surgery echocardiographic examination was focused on the function of the prosthesis leaflets, transvalvular pressure gradient, and aortic regurgitation.
Statistical analysis
Continuous variables are expressed as mean ± SD, and categorical variables as percentages. Bland-Altman plots with an evaluation of systematic bias and 95% confidence intervals (± 1.96 standard deviation of the difference) were used to assess consistency between the surgical and transcatheter prosthesis size selections. For comparison of nonparametric variables, a Spearman rank-order corelation analysis was used, where a p-value < 0.05 was considered statistically significant. Analytics were performed using Statistica version 13.3 from StatSoft.
Results
Of the 265 consecutive patients with aortic stenosis treated with SAVR only 167 patients were eligible for inclusion in this study. The average age of the patients studied was 63 ±10 years, and 91 (54.5%) patients were male. All patients were treated for severe symptomatic aortic stenosis, with 29 (17.3%) cases of patients with a bicuspid aortic valve. Based on the preprocedural transoesophageal echocardiographic examinations, the average aortic valve area was 0.75 ±0.17 cm2, the average mean transvalvular gradient was 57.5 ±17 mm Hg, and the average left ventricular ejection fraction was 62 ±11%.
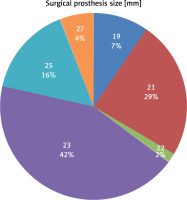
Patients received valve replacements using both biological and mechanical valve prostheses. Sixty-one patients received the St. Jude Medical Regent Valve, 56 the Carpentier-Edwards PERIMOUNT Aortic Heart Valve, 44 the Medtronic Hancock II Tissue Valve, 3 the ATS Aortic Heart Valve, 2 the Carpentier-Edwards PERIMOUNT Magna Ease, and in 1 case the Medtronic Mosaic Tissue Valve. The sizes of prostheses chosen varied from a minimum diameter of 19 mm to maximum diameter of 27 mm (Figure 1). Among patients for whom SAVR was performed, in 12 cases the procedure included mitral valve repair and in 7 cases tricuspid valve annuloplasty was performed. In 22 patients additional aortic bypass grafting was also performed.
Based on the CCT image analysis, the average value of direct measurement of the aortic annulus diameter was 25.4 ±3.0 mm. The average aortic annulus area was 512.7 ±129.0 mm2, and the average aortic diameter calculated, based on the area measurement, was 25.4 ±3.1 mm. The average aortic annulus perimeter was 806.9 ±108.8 mm, and the average aortic diameter calculated, based on the perimeter measurement, was 25.7 ±3.5 m). Aortic valve and annulus calcifications were assessed quantitively and categorized as mild in 29 cases, moderate in 78 cases, and severe in 53 cases. In the CCT images of 7 patients the aortic valve was not calcified at all.
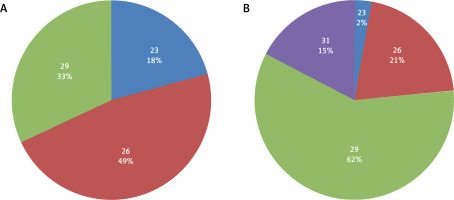
During TAVR sizing selection valves were selected from those available at that time for the Medtronic Core-Valve and for the Edwards SAPIEN XT prostheses. According to the results of the sizing simulation, the whole selection of self-expandable CoreValve prosthesis – 23, 26, 29, and 31 mm and all of the available balloon expandable SAPIEN XT valves – 23, 26, and 29 mm (Figure 2), were used in the study patients. Based on the CTA image assessment, in 18 patients there was no appropriate valve size available for the transcatheter implantation, and according to the TAVR protocol criteria they would be disqualified from transcatheter treatment.
Figure 2
Balloon-expandable (A) and self-expandable (B) valves sizes (mm) selected in TAVR simulation
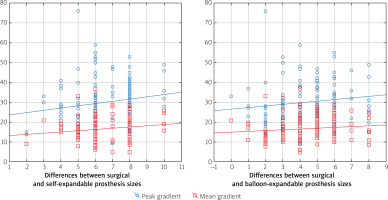
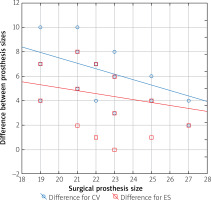
The sizes of the valves recommended by the simulations were larger than valves surgically implanted in 98.6% of patients for self-expanding and in 91.7% of patients for balloon-expandable prostheses. Analysis of the Bland-Altman graphs revealed a systematic difference in the valve size selection between the surgical and transcatheter treatments. Prostheses chosen by surgeons were consistently smaller than valves selected as a result of the TAVR sizing simulation (Figures 3 and 4). The average difference for self-expanding prostheses was 6.4 mm (+1.96 SD = –3.4; –1.96 SD = –9.5) and 4.5 mm (+1.96 SD = –1.1; –1.96 SD = –7.9) for balloon expandable valves. Additionally, a negative correlation was observed for the difference in prosthesis size and size of the valve used by surgeons (Figure 5). The negative correlation was more significant for self-expanding prosthesis selection (R Spearman –0.43; p < 0.05) than for balloon expandable valves (R Spearman –0.17; p < 0.05).
Figure 3
Bland-Altman graph with difference in the valve size selection between SAVR and TAVR with self-expandable prosthesis
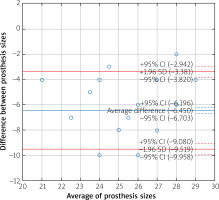
Figure 4
Bland-Altman graph with difference in the valve size selection between SAVR and TAVR with balloon-expandable prosthesis
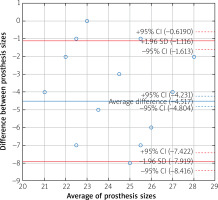
Figure 5
The difference in the prosthesis sizes and the size of the valve used by surgeon
CV – Medtronic CoveValve prosthesis, ES – Edwards Sapien XT prosthesis.
In the postprocedural transthoracic examinations the average maximal transvalvular gradient was 29.4 ±10.3 mm Hg, and the average mean transaortic gradient was 16.3 ±5.9 mm Hg. In 37 patients the mean transaortic gradient post SAVR was higher than 20 mm Hg. Aortic valve regurgitation was assessed as trivial in 72 cases, mild in 24 cases, and moderate in only 1 patient. Analysis showed that transaortic gradients were higher among patients with a smaller prosthesis with an R Spearman correlation of 0.25 (p < 0.05) for a maximum gradient and 0.22 (p < 0.05) for a mean gradient. Additionally, there was a positive correlation between the transaortic gradient and the difference in prosthesis size selection (Figure 6). For the simulation of balloon expandable valves, the correlation was statistically significant for maximum and mean transaortic gradients (R Spearman 0.19 and 0.17, respectively; p < 0.05). In those cases, with selection of self-expandable valves, the correlation was statistically significant only for the maximum transaortic gradient (R Spearman 0.18; p < 0.05).
Discussion
Prosthesis sizing is crucial for the successful invasive treatment of aortic valve stenosis. Inappropriate sizing of the implanted valve may result in prosthesis dysfunction with high transaortic gradients or aortic regurgitation. Suboptimal SAVR or TAVR results with impaired haemodynamic performance significantly affects the clinical outcome and could worsen the long-term prognosis [2] for the patient. Acute mechanical complications, e.g. valve embolization or annular rupture, after implantation of too small or too large prostheses, were also observed [3, 4].
This study revealed that the treatment of the same patient with severe aortic stenosis with 2 alternative invasive procedures – SAVR and TAVR – could result in a different prosthesis size selection. Hypothetical percutaneous treatment of the patients included in the study would result in the implantation of larger valves than the prosthesis used during the SAVR. According to these findings, we could expect that if percutaneously implanted valves achieved their nominal dimensions, the haemodynamic performance after TAVR could be better than after SAVR. This hypothesis corresponds well with our finding that the haemodynamic result is related to the prosthesis size and with the difference between sizes in the chosen surgical and transcatheter prostheses. The transaortic gradients measured postoperatively were higher in the patients with a smaller prosthesis implanted. However, it must be noted that there was only 1 (0.6%) case of moderate aortic regurgitation after SAVR, 24 (14%) cases of a moderate, and 72 (43%) cases of a trivial leak. Based on the large registries with TAVR treatment, we could expect that the aortic regurgitation would be more frequent after TAVR than SAVR.
Analysis, based only on the transaortic velocity measurement from the echocardiographic examination does not allow assessment of the patient-prosthesis mismatch (PPM) phenomenon among the study patients. Nevertheless, with the higher transaortic gradients observed after the implantation of prostheses we could expect a higher frequency of PPM as a reason for the suboptimal haemodynamic result. It was shown in large observational studies that the PPM significantly affects treatment results and patient outcomes [5]. The PPM is observed more frequently after SAVR than after TAVR, with up to 58% frequency after SAVR among patients with a small aortic annulus [6]. After TAVR we could observe PPM in up to one third of patients (32%) in the study with self-expanding prostheses [7] and 11% in the study with balloon-expandable valves [8]. Interestingly, it was shown that the PPM after the implantation of CoreValve and Edwards Sapien prostheses was not related to the native annulus dimensions and prosthesis size, even among patients with small aortic annulus with the minimal diameter of 20 mm for the CoreValve prosthesis [7]. Extended follow-up after TAVR with the balloon-expandable prostheses showed that the occurrence of PPM 12 months after TAVR could be as low as 6%. The comparative study of TAVR vs. SAVR (with stented and stentless valves) revealed a higher frequency of PPM after SAVR (in both subgroups) with the most significant differences among the patients with small native aortic annulus (16–20 mm) [8].
The interpretation of results and conclusions from this study are complicated particularly due to the differences in valve design as well as the lack of standardization in prosthesis labelling. Nominal prosthesis size as given by the manufacturer (“labelled valve size”) is usually the valve’s external diameter (“patient tissue annulus diameter”). In practice, valves have a significantly smaller effective orifice diameter and area, especially in the case of stented valves. The internal part of the valve is usually covered with tissue material which is the same as the leaflet material. This takes 1–2 mm from the stent internal valve diameter to give us a true internal diameter. Additionally, in most of the valves the actual external diameter is larger than the labelled valve size due to the sewing rim surrounding the stent. Taking into account all these construction factors with diameters of stent struts and tissue layers, the true internal diameter of valve is usually 2 mm smaller than the labelled valve size. Smaller differences between the true internal diameter and the labelled valve size are observed in the stentless and the sutureless valve, but these valve types were not used in our study.
In the case of the self-expanding CoreValve prosthesis manufactured by Medtronic the labelled valve size is the same as the inflow part diameter and is significantly larger than the diameter of the constrain part. However, we note that this valve is implanted in a slightly different manner because it is designed as a supra-annular valve. With a self-expanding valve, the true diameter after expansion could be affected by calcifications of the landing zone and native valve as well as an asymmetrical shape of the annulus. The final expansion diameter of self-expanding valves is dependent on radial force of the prosthesis, but the result of implantation could also be optimized with the balloon post dilatation.
The design of the balloon-expandable valves for TAVR is more similar to surgical sutureless valves with small differences between the labelled valve size and the true internal diameter. But as with the self-expanding prosthesis, there are landing zone- and procedure-related factors that determine the final expansion diameter. The native valve calcifications and fibrosis or raphe between leaflets could significantly impair the expansion of the valve. The operator can modify the result of the valve implantation by inflation of the balloon before, during, and after the valve implantation. Based on ex vivo studies, it was shown that the labelled valve size given by Edward Lifesciences corresponds to the stent frame of the valve expanded with the balloon filled with the nominal fluid volume [9]. However, the final expansion diameter of the balloon-expandable prosthesis could be optimized to the desired diameter with an overfilled balloon post dilatation.
The input from an international group of experts resulted in the publication of document containing their consensus opinion regarding the valve characteristics, parametric description, and labelling with graphic illustrations. Labelling according to the consensus would allow for a better understanding and interpretation of the relation between valve sizes and their haemodynamic performance. The published document highlights the importance of taking into consideration the predicted reference effective orifice area for the specific valves during valve sizing. This information combined with data provided by the manufacturer about the risk of PPM in tabular form could be the crucial element of procedural planning and would result in the prevention of PPM [10].
The results of this study, together with current knowledge from the referenced studies, show differences in the procedural planning regarding the valve choice between SAVR and TAVR. Following different procedural paths, we could achieve distinct procedural and clinical results, which is particularly important in specific groups of patients. Patients with the small native aortic annulus could benefit from percutaneous treatment by lowering the risk of PPM after valve implantation [11]. Conversely, patients with a large aortic annulus are at higher risk of aortic regurgitation after TAVR; therefore, SAVR [12] could be considered as a preferred treatment. In the clinical scenarios where patients are eligible for SAVR and TAVR optimal management should be based on a personalised individual and comprehensive assessment. The cardiac imaging results with the measurements indexed for body surface area together with predicted risk of PPM for available valves are crucial for a tailored aortic stenosis treatment [13].
Conclusions
This study shows that there is a discrepancy between the results of the SAVR and TAVR sizing process in patients treated for severe aortic stenosis. In patients treated percutaneously the nominal sizes of prostheses are larger, and therefore patients would potentially achieve larger true internal diameter and effective orifice area. Randomized trials could allow for an assessment as to how the discrepancy in valve sizing would affect clinical outcomes with a particular focus on PPM, aortic regurgitation, and prosthesis durability. However, to perform a reliable comparison of valve sizes used in SAVR and TAVR it is necessary to get precise standardized prosthesis labelling by manufacturers.
It is of paramount importance to utilize the opportunities of procedure planning and optimization based on the cardiovascular imaging results. The CCT examination together with the echocardiographic examination should be the primary assessment tools for patients with aortic stenosis. The value of the CCT assessment is much more than just diagnosing the coronary artery disease during SAVR planning. CCT is now routinely used for TAVR planning, but it could also be a valuable part of SAVR qualification. Operators should be encouraged to use cardiac imaging results for valve sizing before SAVR and make a comprehensive assessment with the echocardiographic and CCT results indexed for body surface area. The results of the CCT examination used by the heart team could allow for personalised treatment for patients considering the conditions and limitations related to SAVR and TAVR.
Our study has several limitations. This study was designed as single-centre retrospective analysis of patients treated in 2012–2016. Patients included in the study had undergone SAVR, and it was not possible to assess how many of them would be eligible candidates for TAVR. The calculation of prosthesis sizing was based only on the CCT images without the possibility of angiographic annulus diameter verification. The results of echocardiographic examinations did not allow assessment of the effective orifice area after SAVR, and thus it was impossible to establish the PPM diagnosis and its influence on the patients prognosis.