Introduction
Hepatocellular carcinoma (HCC) is the most important primary liver cancer and is the world’s 6th most frequent cancer and the 3rd leading cause of cancer related death [1]. Cirrhosis is the commonest trigger for HCC, which can complicate most of the causes of cirrhosis [2].
Early detection of HCC is crucial for minimizing HCC-related mortality since many patients are diagnosed late in the course of their HCC and thus miss out on potential therapies [3].
The European Association for the Study of the Liver(EASL) recommends that patients with liver cirrhosis undergo hepatic ultrasound, which is an indirect diagnostic technique with limited ability to distinguish HCC from non-neoplastic lesions and is heavily dependent on operator expertise [4].
Serum α-fetoprotein (AFP) is insufficient as a biomarker for HCC since its sensitivity ranges from 40% to 65%. AFP levels are rarely increased in a significant proportion of people with early-stage, potentially curable HCC. Furthermore, AFP levels may rise transiently, intermittently, or chronically in patients with viral hepatitis who do not have HCC [5].
CXCL9 is a short cytokine which represents a part of the CXC chemokine family that causes tissue extravasation by stimulating chemotaxis and promoting leukocyte differentiation and proliferation by binding with the chemokine receptor CXCR3 [6].
CXCL9 facilitated the invasiveness of CD133+ hepatic malignant cells by stimulating the phosphorylated extracellular signal-regulated kinase 1/matrix metalloproteinase 2/matrix metalloproteinase 9 pathway [7]. Lan et al. discovered that by overexpressing phosphatidylinositol-3,4,5-trisphosphate Rac exchanger 2, CXCL9 improves the invasion potential of HCC [8].
Pentraxin 3 corresponds to the pentraxin superfamily, which includes both short pentraxins such as C-reactive protein and serum amyloid P and long pentraxins such as pentraxin 3 [9]. It participates in the innate immune system and inflammation by controlling the complement system and also it has an important role in tissue remodelling and repair [10, 11].
Pentraxin 3 expression has been associated with the development of cancers such as prostate cancer, gastric cancer, liposarcoma, pancreatic carcinoma, lung carcinoma, and glioma. However, little is known about pentraxin 3’s role in HCC [10].
The aim of this study was to evaluate serum CXCL9 and pentraxin 3 as diagnostic markers of HCC among cirrhotic HCV patients.
Material and methods
This study included 90 individuals admitted to the Tropical Medicine Department of Alexandria Main University Hospital, who were divided into 3 groups: group I (n = 30) patients with HCV induced liver cirrhosis without HCC, group II (n = 30) patients with HCV induced liver cirrhosis with HCC, and group III (n = 30) healthy subjects.
All patients underwent complete history taking and clinical assessment. Laboratory investigations included: complete blood count, liver function assays, renal function tests, C-reactive protein, fasting blood sugar, viral hepatitis markers B and C, PCR for HCV RNA and serum a-fetoprotein. All cirrhotic cases were assigned to Child-Pugh classification, and HCC cases were assigned to Barcelona Clinic Liver Cancer (BCLC) classification. Human CXCL9 ELISA kit and Human Pentraxin 3 ELISA kit were used to measure serum CXCL9 and serum pentraxin 3 respectively. All patients were previously treated for HCV by directacting antiviral drugs and achieved sustained virological response (SVR), which was confirmed by a negative polymerase chain reaction (PCR) for HCV at the time of the study. All groups had abdominal ultrasound, and a triphasic computed tomography (CT) scan was ordered to diagnose HCC by showing the typical radiological pattern of arterial hyperenhancement and delayed venous washout.
Exclusion criteria
Patients with HIV infection, chronic HBV infection, any cause of chronic hepatitis other than HCV, malignancies other than HCC, diabetes mellitus, kidney disease, heart disease, autoimmune disease and active infection were excluded from this research.
Statistical analysis
Sample size was calculated using Power Analysis and Sample Size Software (PASS 2020; NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/pass). A minimal total hypothesized sample size of 90 eligible patients recruited from Main Alexandria University Hospitals (30 per group) is needed to study the value of serum CXCL9 and pentraxin 3 as a diagnostic marker of hepatocellular carcinoma among cirrhotic HCV patients, taking into consideration a 95% confidence level and 80% power using the chi-square (χ2) test [12-14].
The IBM SPSS software program version 20.0 was used to analyse the data. Chi-square test, Fisher’s exact test, Student t-test, F-test (ANOVA), Mann-Whitney test, Kruskal-Wallis test, and Spearman coefficient were the statistical tests utilized. Plotting sensitivity (TP) on the Y axis versus 1-specificity (FP) on the X axis at various cut-off levels yielded a receiver operating characteristic curve (ROC). The diagnostic performance of a test is measured by the area under the ROC curve.
Ethical approval
All participants signed a written informed consent form. This study was authorized by the Alexandria University Faculty of Medicine’s ethical committee and is in agreement with the Helsinki Declaration of 1975. Committee reference No.: 0201402. Date: 15 October 2020. IRB No.: 00012098. FWA No.: 00018699.
Results
Table 1 compares the demographics of liver cirrhosis and HCC groups with the control group in relation to age and gender.
Table 1
Comparison of the three groups analysed based on demographic data
Serum AFP levels in HCC patients were statistically significantly higher than in cirrhotic patients and the control group (p < 0.001, p < 0.001). However, there was no statistically significant difference between the cirrhosis and control groups (p = 0.222) (Table 2).
Table 2
Comparison of the three groups analysed based on tumour markers
[i] SD – standard deviation, F – F for one-way ANOVA test, pairwise comparison between each 2 groups was done using a post hoc test (Tukey), H – H for Kruskal-Wallis test, pairwise comparison between each 2 groups was done using a post hoc test (Dunn’s test for multiple comparisons), p – p value for comparing between the three studied groups, p1 – p value for comparing between cirrhosis and HCC, p2 – p value for comparing between cirrhosis and control, p3 – p value for comparing between HCC and control, * statistically significant at p ≤ 0.05
Serum CXCL9 values were statistically significantly higher in HCC patients compared to cirrhotic patients and the control group (p < 0.001, p < 0.001). Also, they were statistically significantly higher in the cirrhosis group than the control group (p = 0.005) (Table 2).
Serum pentraxin 3 levels were statistically significantly higher in patients with HCC than in patients with cirrhosis and the control group (p < 0.001, p < 0.001). Also, they were statistically significantly higher in the cirrhosis group than the control group (p < 0.001) (Table 2).
Serum CXCL9 concentrations were statistically significantly higher in HCC patients with different parameters such as infiltrative type (p = 0.038), lymph node metastasis (p = 0.007), distant metastasis (p = 0.002) and portal vein thrombosis (p = 0.001). The only exceptions were tumour number (p = 0.580) and BCLC classification (p = 0.218) (Table 3).
Table 3
Relation between CXCL9 and pentraxin 3 with different tumour parameters in hepatocellular carcinoma (HCC) group (n = 30)
Serum pentraxin 3 levels were statistically significantly higher in HCC patients with various parameters such as tumour number (p = 0.022), lymph node metastasis (p = 0.001), distant metastasis (p < 0.001) and portal vein thrombosis (p = 0.022) except infiltrative type (p = 0.129) and BCLC classification (p = 0.200) (Table 3).
The largest tumour diameter ranged between 1.50 and 18.0 cm with a mean of 7.49 ±5.08 cm and there was significant positive correlation between tumour size and CXCL9, as well as pentraxin 3 (Table 3). The dependence between the tumour size and the cut-off for diagnosing HCC using CXCL9 and pentraxin 3 is presented in Table 4.
Table 4
Relation between cut-off for diagnosing hepatocellular carcinoma (HCC) using CXCL9 and pentraxin 3 and tumour size in HCC group (n = 30)
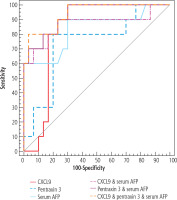
The diagnostic performance, cut-off point, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for discriminating HCC from liver cirrhosis for CXCL9, pentraxin 3 and AFP are shown in Table 5. ROC curves were generated to evaluate the cut-off points for CXCL9, pentraxin 3 and AFP for predicting the probability for HCC, as illustrated in Figure 1.
Table 5
Validity (AUC, sensitivity, specificity, PPV, NPV) for CXCL9, pentraxin 3 and serum AFP to discriminate hepatocellular carcinoma (HCC) patients (n = 30) from cirrhosis patients (n = 30)
Fig. 1
ROC curve for CXCL 9 (ng/l), pentraxin 3 (ng/ml) and serum AFP (ng/ml) to discriminate HCC patients (n = 30) from cirrhosis patients (n = 30)
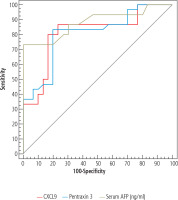
The combinations CXCL9 and AFP, pentraxin 3 and AFP, and CXCL9, pentraxin 3 and serum AFP have their diagnostic performance, sensitivity, specificity, PPV and NPV for distinguishing HCC from liver cirrhosis presented in Table 6 and the ROC curves in Figure 2.
Table 6
Validity (AUC, sensitivity, specificity, PPV, NPV) for combined CXCL9, pentraxin 3 and serum AFP to discriminate hepatocellular carcinoma (HCC) patients (n = 30) from cirrhosis patients (n = 30)
Fig. 2
ROC curve for combined CXCL 9 (ng/l), pentraxin 3 (ng/ml) and serum AFP (ng/ml) to discriminate HCC patients (n = 30) from cirrhosis patients (n = 30)
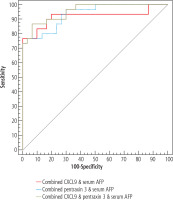
The diagnostic performance, cut-off point, sensitivity, specificity, PPV, NPV for CXCL9, pentraxin 3 and serum AFP alone and in combinations to discriminate early HCC patients (BCLC A+B) from cirrhosis patients are shown in Table 7 and the ROC curves in Figure 3.
Table 7
Validity (AUC, sensitivity, specificity, PPV, NPV) for CXCL9, pentraxin 3 and serum AFP alone and in combinations to discriminate early hepatocellular carcinoma (HCC) patients (BCLC A+B) (n = 10) from cirrhosis patients (n = 30)
Discussion
Hepatocellular carcinoma is one of the world’s most frequent malignancies with a high death rate, and survival rates as low as 1% in untreated individuals after 2 years. With more than two-thirds of patients diagnosed at late stages of illness, identifying the most valuable biomarkers to serve as additions or alternatives for AFP in HCC diagnosis, and even an appropriate tool for assessing tumour spread and patient prognosis, is critical, since these markers could indeed guide clinical decision-making concerning HCC treatment [15].
Serum CXCL9 and serum pentraxin are studied in our research as potential markers for accurate and early diagnosis of HCC.
In this research, serum CXCL9 levels were considerably higher in the HCC group when compared to the cirrhotic and control groups, which is compatible with Wang et al. [16] data of higher CXCL9 expression in HCC tissues. Furthermore, the cut-off for diagnosing HCC using serum CXCL9 was 296 ng/l, with a sensitivity of 86.67%, a specificity of 76.67%, and an area under the ROC curve (AUROC) of 0.812.
Additionally, serum pentraxin 3 levels were considerably higher in the HCC group than in the cirrhotic and control groups, which is consistent with Song et al. [14] who found higher pentraxin 3 expression in HCC tissues. Furthermore, the cut-off for serum pentraxin 3 to diagnose HCC was 5.36 ng/ml, with a sensitivity of 83.33%, a specificity of 80.0% and an AUROC of 0.804.
Several combinations were significant in distinguishing cirrhotic cases from HCC, including CXCL9 and AFP, pentraxin 3 and AFP, and CXCL9, pentraxin 3 and AFP, with increasing AUROC (0.918, 0.934, 0.954 respectively) and sensitivity (90.0%, 93.33%, 96.67% respectively) but decreasing specificity (83.33%, 73.33%, 70.0% respectively).
The use of serum CXCL9 and serum pentraxin 3 as early HCC markers at the same cut-off points was tested and our study found that serum CXCL9 levels and serum pentraxin 3 levels alone and in the combinations CXCL9 and AFP, pentraxin 3 and AFP, and CXCL9, pentraxin 3 and AFP were statistically significantly higher in the early HCC group (BCLC A+B) than in the cirrhotic group. However, more studies are needed to confirm these results because of the small size of the early HCC group (10 patients).
Our study revealed a significant positive correlation between serum CXCL9 and various HCC parameters such as tumour size, infiltrative type, lymph node metastases, distant metastases, and portal vein thrombosis, which was consistent with Lan et al. [8], who found that CXCL9 increases HCC invasion ability. BCLC and tumour number were the only HCC parameters in our analysis that were not correlated with CXCL9.
The current research found a significant relationship between serum pentraxin 3 and different HCC parameters such as tumour size, tumour number, lymph node metastases, distant metastases, and portal vein thrombosis, which was in agreement with Song et al. [14], who found higher pentraxin 3 expression in more aggressive HCCs. BCLC and infiltrative pattern were the only variables in our analysis that were not correlated with pentraxin 3.
Conclusions
The significantly elevated levels of serum CXCL9 and serum pentraxin 3 in HCV patients with HCC could support their use as early diagnostic markers for HCC, either alone or in addition with each other or with serum AFP, allowing for quick intervention, at a time frame allowing for radical treatment of HCC. Furthermore, the significant high values of both markers in patients with larger tumour size, lymph node metastasis, portal vein thrombosis and distant metastasis could suggest their use as an indicator of HCC aggressiveness as well as a guide to the best treatment modality for HCC as additional tools with BCLC and AFP. However, more studies are needed to evaluate the role of these markers in HCC caused by other aetiologies of liver cirrhosis such as HBV and NAFLD.