Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death worldwide and the 5th most prevalent one overall [1]. Almost 90% of primary liver cancers are HCCs [2]. Early diagnosis of HCC is essential to reduce HCC-related deaths [3].
Abdominal ultrasonography is not a direct diagnostic method as it has limited ability to differentiate HCC from non-neoplastic lesions. The European Association for the Study of the Liver (EASL) proposes that those with liver cirrhosis should undergo abdominal ultrasound [4]. Serum α-fetoprotein (AFP) is an important biomarker for HCC, yet it has 40-65% sensitivity, and its levels in early HCC are rarely elevated [5].
Mac-2 binding protein is an extracellular matrix cell adhesive glycoprotein produced as a galectin 3 ligand. Several types of liver cells produce it. Hepatic stellate cells create the glycoprotein known as Mac-2 binding protein glycosylation isomer (M2BPGi). It facilitates fibrogenesis by acting as a messenger between Kupffer cells and hepatic stellate cells [6].
The degree of liver fibrosis may become a key element in the course of the illness. The degree of fibrosis has been determined using a variety of noninvasive techniques, such as serum biomarkers and ultrasound-based tests [7].
Recent research suggests that the serum biomarker M2BPGi may be related to fibrosis in addition to the risk of developing HCC in individuals with chronic hepatitis C [8]. The aim of this study was to assess serum Mac-2 BPGI among cirrhotic hepatitis C virus (HCV) patients as diagnostic indicators of HCC.
Material and methods
This study included 90 patients divided into two groups: group I comprising 45 HCV cirrhotic patients without HCC and group II comprising 45 HCV cirrhotic patients with HCC. All the individuals involved in this research were cirrhotic and all of them were successfully treated for HCV and achieved sustained virologic response (SVR). However, during their routine follow-up after achieving SVR, some of them developed HCC (group II).
A thorough history was taken followed by a clinical evaluation. Complete blood count (CBC), liver, kidney functions tests, fasting blood sugar, serum cholesterol and triglycerides, viral hepatitis markers B, C, HCV PCR and serum AFP were among the laboratory evaluations that were done. Every case of cirrhosis was assessed for a Child-Pugh classification, while HCC patients were assessed for a BCLC classification.
Serum Mac-2 BPGI was measured using a human Mac-2 BPGI enzyme-linked immunosorbent assay (ELISA) kit. A negative HCV PCR at the time of the study confirmed that all patients who had previously had treatment for HCV with direct-acting antiviral medications had also achieved SVR.
Both groups underwent abdominal ultrasound and triphasic CT was conducted in group II for diagnosis of HCC by demonstrating the characteristic radiological findings of arterial hyperenhancement and delayed venous washout.
The kit measures the amount of human M2BPGI in samples using a double-antibody sandwich ELISA kit provided by Biokit catalogue number 201-12-8411 China.
M2BPGI was added to a monoclonal antibody enzyme well that had already been coated with the human M2BPGI monoclonal antibody.
Inclusion criteria
Group I comprised 45 HCV cirrhotic patients without HCC and group II comprised 45 HCV cirrhotic patients with HCC. Neither group had other causes of cirrhosis such as hepatitis B virus (HBV), autoimmune causes or other causes of M2BPGi elevation such as biliary abnormalities and bile duct injury.
Exclusion criteria
Patients who had liver cirrhosis other than HCV (e.g. HBV and autoimmune causes) or other causes of M2BPGi elevation such as biliary abnormalities and bile duct injury such as primary sclerosing cholangitis and primary biliary cirrhosis were not included in the study.
Statistical analysis
Using the Power Analysis and Sample Size software (PASS 2020; NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/pass), the sample size was determined. To investigate the utility of serum Mac-2 BPGI as a diagnostic marker of HCC in cirrhotic HCV patients, a minimum total anticipated sample size of 90 suitable patients selected from Main Alexandria University Hospitals (45 per group) was required, taking into account a 95% confidence level and 80% power utilizing the chi-square (χ2) test.
The data were analyzed using IBM SPSS version 20.0 software. The statistical tests used were the Mann-Whitney, χ2, Fisher’s exact, Student’s t-test, F-test (ANOVA), Kruskal-Wallis, and Spearman coefficient. A receiver operating characteristic curve (ROC) was produced by plotting sensitivity (TP) on the Y axis and 1-specificity (FP) on the X axis at different cut-off values. The area under the ROC curve is used to evaluate how well a test functions as a diagnostic tool.
Ethical approval
A formal informed consent form was signed by each participant. The Alexandria University Faculty of Medicine’s ethical committee approved this study and it is in agreement with the Helsinki Declaration of 1975. Committee reference No. 0107161, Date: 21 April 2022, IRB No. 00012098, FWA No. 00018699.
Results
Regarding demographic data, there was no statistically significant difference between groups regarding sex (p = 0.090). In group I patients there were 16 males (35.6%) and 29 females (64.4%), while those of group II were 24 males (53.3%) and 21 females (46.7%).
Regarding the age of patients, in group I it ranged from 43 to 75 years, with a mean of 59.84 ±7.78 years, while that of group II ranged from 45.0 to 78.0 years with a mean of 61.47 ±7.10. There was no statistically significant difference between groups regarding age (p = 0.304) (Table 1).
Table 1
Comparison between the two studied groups according to demographic data
Regarding symptoms, there was a statistically significant difference between group I and group II as regards easy fatigue, yellow discoloration of the eye, weight loss, hematemesis, and melena, being higher in group 2 (p < 0.001), but there was no statistically significant difference as regards abdominal distension and encephalopathy (p value = 0.832, 0.163, respectively).
Regarding the signs, there was a statistically significant difference between group I and group II as regards jaundice, hepatomegaly (p < 0.001) and splenomegaly (p = 0.044), but there was no statistically significant difference between group I and group II as regards ascites and LL edema, with p values of 1.000 and 1.000, respectively (Table 2).
Table 2
Comparison between the two studied groups according to symptoms and signs
| Parameter | Group I (n = 45) | Group II (n = 45) | χ2 | p | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Symptoms | ||||||
| Easy fatigue | 11 | 24.4 | 31 | 68.9 | 17.857* | < 0.001* |
| Abdominal distension | 24 | 53.3 | 25 | 55.6 | 0.045 | 0.832 |
| Yellow discoloration | 2 | 4.4 | 17 | 37.8 | 15.011* | < 0.001* |
| Weight loss | 3 | 6.7 | 40 | 88.9 | 60.965* | < 0.001* |
| Hematemesis | 10 | 22.2 | 27 | 60.0 | 13.264* | < 0.001* |
| Melena | 10 | 22.2 | 27 | 60.0 | 13.264* | < 0.001* |
| Encephalopathy | 16 | 35.6 | 10 | 22.2 | 1.947 | 0.163 |
| Signs | ||||||
| Jaundice | 2 | 4.4 | 17 | 37.8 | 15.011* | < 0.001* |
| Ascites | 24 | 53.3 | 24 | 53.3 | 0.000 | 1.000 |
| LL edema | 24 | 53.3 | 24 | 53.3 | 0.000 | 1.000 |
| Splenomegaly | 37 | 82.2 | 43 | 95.6 | 4.050* | 0.044* |
| Hepatomegaly | 4 | 8.9 | 25 | 55.6 | 22.436* | < 0.001* |
Regarding the laboratory investigations, there was a statistically significant difference between groups, being higher in group II, as regards platelets, white blood cells, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total and direct bilirubin, with p values of 0.015, 0.002, < 0.001, < 0.001, < 0.001, < 0.001, respectively. There was no statistically significant difference between groups as regards hemoglobin, serum albumin, prothrombin time (PT), international normalised ratio (INR), blood urea, or serum creatinine, with p values of 0.059, 0.125, 0.262, 0.152, 0.500, 0.052, respectively (Table 3).
Table 3
Comparison between the two studied groups according to laboratory investigations
Regarding AFP, in group I it was in the range 1.10-29.0 ng/ml with a mean of 6.15 ±6.33 ng/ml and in group II it was in the range 2.0-500.0 ng/ml with a mean of 48.33 ±78.05 ng/ml. There was a statistically significant difference between groups, being higher in group II (p < 0.001) (Table 4).
Table 4
Comparison between the two studied groups according to serum α-fetoprotein (AFP)
| Serum AFP | Group I (n = 45) | Group II (n = 45) | U | p |
|---|---|---|---|---|
| Min.-Max. | 1.10-29.0 | 2.0-500.0 | 185.00* | < 0.001* |
| Mean ±SD | 6.15 ±6.33 | 48.33 ±78.05 | ||
| Median (IQR) | 3.40 (2.0-5.0) | 20.0 (18.0-60.0) |
Regarding serum M2BPGi, in group I it was in the range 50.24-953.4 with a mean of 219.9 ±150.5 and in group II it was in the range 92.08-1557.6 with a mean of 938.7 ±431.2. The difference between the two groups was statistically significant, with group II showing a higher value (p < 0.001) (Table 5).
Table 5
Comparison between the two studied groups according to serum M2BPGi
| Serum M2BPGi | Group I (n = 45) | Group II (n = 45) | U | p |
|---|---|---|---|---|
| Min.-Max. | 50.24-953.4 | 92.08-1557.6 | 133.00* | < 0.001* |
| Mean ±SD | 219.9 ±150.5 | 938.7 ±431.2 | ||
| Median (IQR) | 184.9 (140.1-227.2) | 952.7 (595.1-1290.9) |
Regarding the distribution of the studied cases according to radiological findings in group II (with HCC) (Table 6).
Table 6
Distribution of the studied cases according to radiological findings in group II (with HCC) (n = 45)
| Parameter | n | % | Serum M2BPGi | Test of Sig. | p | |
|---|---|---|---|---|---|---|
| Median (Min.-Max.) | Mean ±SD | |||||
| Portal vein invasion | ||||||
| No | 18 | 40.0 | 444.3 (92.1-877.3) | 487.9 ±259.2 | U = 1.000* | < 0.001* |
| Yes | 27 | 60.0 | 1272.8 (871.9-1557.6) | 1239.2 ±190.4 | ||
| Lymph node metastases | ||||||
| No | 34 | 75.6 | 867.9 (92.1-1557.6) | 849.9 ±448.4 | U = 96.00* | 0.015* |
| Yes | 11 | 24.4 | 1275.1 (871.9-1505.5) | 1213.1 ±212.6 | ||
| Tumor number | ||||||
| 1 | 15 | 33.3 | 433.3 (92.1-952.7) | 476.7 ±272.8 | H = 28.434* | < 0.001* |
| 2 | 2 | 4.4 | 491.3 (254.8-727.8) | 491.3 ±334.5 | ||
| 3 | 18 | 40.0 | 1243.7 (858.5-1505.5) | 1199.7 ±190.8 | ||
| > 3 | 10 | 22.2 | 1273.6 (743.3-1557.6) | 1251.4 ±258.8 | ||
| Tumor size largest dimension (Max.) | ||||||
| Min.-Max. | 2.0-8.0 | – | – | – | – | |
| Mean ±SD | 6.11 ±1.92 | – | – | |||
| Median (IQR) | 7.0 (5.0-8.0) | – | – | |||
| BCLC | ||||||
| A | 9 | 20.0 | 433.3 (92.1-877.3) | 477.0 ±263.3 | H = 31.441* | < 0.001* |
| B | 9 | 20.0 | 455.2 (215.5-858.5) | 498.8 ±270.6 | ||
| C | 15 | 33.3 | 1275.1 (871.9-1505.5) | 1237.9 ±190.1 | ||
| D | 12 | 26.7 | 1235.5 (878.4-1557.6) | 1240.9 ±199.2 | ||
As regards portal vein invasion, in the HCC group, 18 patients had no portal vein invasion, their serum M2BPGI levels ranged between 92.08 and 877.3 with a mean of 487.9 ±259.2, while 27 patients had portal vein invasion and their serum M2BPGI levels ranged from 871.9 to 1557.6 with a mean of 1239.2 ±190.4. The difference in mean level of serum M2BPGI was statistically significant, being higher in patients with portal vein invasion than those without (p < 0.001).
As regards lymph node metastases, in the HCC group, 34 patients had no lymph node metastases; their serum M2BPGI level range was 92.08-1557.6 (mean 849.9 ±448.4), while 11 HCC patients had lymph node metastases and their serum M2BPGI level range was 871.9-1505.5 (mean 1213.1 ±212.6). The mean level of serum M2BPGI was statistically significantly higher in patients with lymph node metastases than in those without (p = 0.015).
As regards tumor number, in the HCC group, 15 patients had a single lesion; their serum M2BPGI level range was 92.08-952.7 with a mean of 476.7 ±272.8; 2 patients had two lesions; their serum M2BPGI level range was 254.8-727.8 with a mean of 491.3 ±334.5; 18 patients had three lesions; their serum M2BPGI level range was 858.5-1505.5 with a mean of 1199.7 ±190.8; 10 patients had more than three lesions; their serum M2BPGI level range was 743.3-1557.6 with a mean of 1251.4 ±258.8. There was statistically significant variation between mean values of serum M2BPGI in the four groups being higher in patients who had more than three lesions (p < 0.001).
As regards tumor size, there was a clear positive relation between it and serum M2BPGI, with a p value < 0.001.
As regards Barcelona Clinic Liver Cancer (BCLC) staging, in the HCC group, serum M2BPGI levels were found to be the lowest in BCLC A patients, where they were in the range 92.08-877.3, mean 477.0 ±263.3, followed by patients with BCLC B where they were in the range 215.5-858.5, mean 498.8 ±270.6, followed by BCLC D patients where they were in the range 878.4-1557.6, mean 1240.9 ±199.2, followed by patients with BCLC C, where they were in the range 871.9-1505.5, mean 1237.9 ±190.1. There was a statistically significant difference between mean values of serum M2BPGI in the four groups, being higher in group C (p < 0.001).
As regards Child-Pugh score, group I contained 18 patients with Child class A (compensated cirrhosis), 25 patients were Child B and 2 patients were Child class C (decompensated cirrhosis), with Child-Pugh scores ranging from 5 to 10 and a mean value of 6.87 ±1.38, while group II contained 16 patients with Child class A (compensated cirrhosis), 17 patients with Child class B and 12 patients with Child class C (decompensated cirrhosis) with scores ranging from 5 to 13 and a mean value of 7.76 ±2.13. There was a statistically significant difference regarding Child scores of both groups, being higher in group II (p = 0.021). This means that group II patients’ hepatic state was more decompensated than that of group A (Table 7).
Table 7
Comparison between the two studied groups according to Child-Pugh score
| Child Pugh score | Group I (n = 45) | Group II (n = 45) | Test of Sig. | p | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| A | 18 | 40.0 | 16 | 35.6 | χ2 = 8.784* | 0.012* |
| B | 25 | 55.6 | 17 | 37.8 | ||
| C | 2 | 4.4 | 12 | 26.7 | ||
| Min.-Max. | 5.0-10.0 | 5.0-13.0 | t = 2.349* | 0.021* | ||
| Mean ±SD | 6.87 ±1.38 | 7.76 ±2.13 | ||||
| Median (IQR) | 7.0 (6.0-7.0) | 7.0 (6.0-10.0) | ||||
The Child-Pugh score is a scoring system classifying the degree of liver cirrhosis decompensation, depending on evaluation of patients’ serum albumin, ascites, hepatic encephalopathy, total bilirubin and prothrombin activity. Patients with Child class A (compensated cirrhosis) = 5-6 points, Child class B = 7-9 points and Child class C = 10-15 points (decompensated cirrhosis).
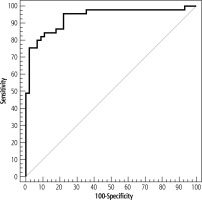
Regarding the Mac-2 BPGI’s diagnostic performance, cut-off point, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), for separating HCC from liver cirrhosis (Table 8).
Table 8
Prognostic performance for serum M2BPGi to discriminate patients with HCC (n = 45) from patients without HCC (n = 45)
| AUC | p | 95% CI | Cut off | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| Serum M2BPGi | 0.934 | < 0.001* | 0.881-0.987 | > 227.551 | 95.56 | 77.78 | 81.13 | 94.59 |
The cut-off points for Mac-BPGI for predicting the likelihood of HCC were evaluated using the ROC curve (Fig. 1).
Discussion
Among all malignancies, HCC is one of the most prevalent, with a high death rate. Our research assessed serum Mac-2 BPGI as a possible marker for early diagnosis of HCC in HCV cirrhotic patients.
In our study, serum M2BPGI was found to be significantly higher in the HCC group compared to the non-HCC group, which is in agreement with Yugawa et al. [9]. Moreover, the cut-off for serum M2BPGI to diagnose HCC was 227.5 ng/l, with sensitivity of 95.56%, specificity of 77.78%, PPV of 81.13%, and NPV of 94.59%.
A study done by Yamasaki et al. [10] revealed that the cumulative 5-year incidence of HCC was 77% in patients who had chronic HCV infection and high M2BPGi, compared to 31.6% in those with low M2BPGI, and this was in agreement with the results of our study.
Another study done by Su et al. [11] evaluated M2BPGi’s capability to anticipate HCC in the subset of patients (n = 68) with the follow-up lasting more than 4 years. Twenty patients had HCC; the AUROC (area under the ROC curve) was 0.79.91; the PPV and NPV were 67% and 83%, respectively, with a sensitivity of 58% and a specificity of 90%, and this was in agreement with the results of our study.
There are also other studies which included assessment of Mac-2 BPGI in cirrhotic patients infected with HBV to anticipate HCC in those patients [12, 13].
In a study done by Liu et al. [12] the prevalence of individuals with high M2BPGi levels increased as samples were collected closer to the diagnosis of HCC. The sensitivity, specificity, PPV, and NPV to predict HCC in 1-2 years were reported to be 45%, 95%, 50%, and 94%, respectively, and this was in agreement with the results of our study.
A study done by Mak et al. [13] also showed that patients with HCC had considerably higher median M2BPGi levels than those without HCC (p = 0.001) with 91.7% sensitivity and 80.8% specificity. M2BPGi produced an AUROC of 0.883, and this was in agreement with the results of our study.
Another study done by Nagata et al. [14] also clarified that real-time monitoring of the blood M2BPGi level for chronic HCV patients could be a meaningful screening tool for determining the risk of HCC, according to research that found serum M2BPGi levels in HCC patients were considerably higher than those without HCC (p = 0.033). Hepatocarcinogenesis could be linked to M2BP and its glycan structure and this was in agreement with the results of our study.
Our research revealed a statistically significant difference regarding Child-Pugh score between groups, being significantly higher in the HCC group, which agrees with the results of the study done by Zhao et al. [15].
In our study, the HCC group had significant positive correlation between serum AFP and serum M2BPGi, which is compatible with the study done by Yugawa et al. [9].
The current work demonstrated a considerable positive correlation between serum M2BPGI and tumor size; serum M2BPGI significantly increased in relation to tumor size, which was in agreement with the study done by Yugawa et al. [9].
The current study showed a significant correlation between serum level of M2BPGI and portal vein thrombosis, that agrees with Tak et al. [16], who stated that M2BPGi is distinctive in that its level corresponds with both the likelihood of developing HCC and the prognosis following treatment for HCC, and this was in agreement with the results of our study.
As regards lymph node metastases, our study revealed a considerable correlation between serum M2BPGI levels and lymph node metastases, which agrees with the study done by Yugawa et al. [9].
As regards number of lesions, the current study showed revealed a significant correlation between serum M2BPGI levels and the number of tumor lesions, which agrees with the study done by Tak et al. [16].