Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD). The disease is characterized by unpredictable periods of remissions and exacerbations, during which the physical and mental well-being of the patient can be significantly impaired. Symptoms of active disease are rectal bleeding, diarrhoea, and rectal urgency. These symptoms reflect disease activity and the extent of inflammation in the colon. The goal of UC treatment is to achieve clinical remission without the need for the use of glucocorticosteroids, which remain the primary class of drugs to induce remission at various stages of treatment [1]. To reduce the risk of adverse events during the use of systemic corticosteroids, budesonide MMX® has been introduced into the therapy of UC. Budesonide is a low bioavailability steroid, and the MMX® technology allows for continuous release of the active molecule along the colon [2]. In the CORE I and CORE II trials, the clinical and endoscopic remission of budesonide MMX® 9 mg in mild-to-moderate UC was proven. The incidence of adverse events in the budesonide MMX® group was comparable to that in the placebo group [3, 4]. In a similar population of patients within the CONTRIBUTE trial, budesonide MMX® was used in the add-on therapy to 5-ASA formulations and showed higher clinical and endoscopic remission compared to the placebo group [5]. Currently budesonide MMX® is registered for induction of remission in adult patients with mild-to-moderate UC, where 5-aminosalicylic acid (5-ASA) treatment is not sufficient [6]. Randomised clinical trials require strict adherence to a study protocol.
Aim
It is therefore important to investigate the effectiveness and safety of budesonide MMX for the treatment of active mild-to-moderate UC in a real-life setting [7].
Material and methods
This report presents the results of Polish patients who took part in a multi-centre, international, observational study: CORE Practice. The study was registered on www.clinicaltrials.gov (https://clinicaltrials.gov/, identifier NCT02586259). The relevant Ethics Committee approved the study according to EU and Polish regulations. The included patients were over 18 years old, recruited in outpatient care. Current flares of mild-to-moderate UC were treated to induce remission with budesonide MMX® 9 mg when 5-ASA treatment was not sufficient. The patient received full information about participation in the study, and once they understood its principles, they signed an informed consent form to participate in the study. The criteria for exclusion from the study were severe UC, treatment of current flare with antibiotics and/or steroids, history of total or sub-total colectomy, hypersensitivity to the active substance, and current participation in an interventional trial. Reporting of adverse events (AE) was carried out in accordance with the legal regulations in Poland with the CIOMS form availability [8].
The duration of the study was 8 weeks. Data from observations were collected during 2 visits. The medical history data included gender, age, smoking, extension of colon inflammation, and intolerance to 5-ASA or steroids. UC medical history data in the last 12 months included the number of UC flares, induction cycles of oral 5-ASA, use of rectal/oral steroids, and the occurrence of adverse events associated with steroids. Clinical response, quality of life, and satisfaction with treatment were evaluated during the 2nd visit. The clinical response to the therapy was assessed using clinical sub-score of the Ulcerative Colitis Disease Activity Index (UCDAI) (rectal bleeding-RF, stool frequency-SF, physician global assessment-PGA) [9]. The Short Inflammatory Bowel Disease Questionnaire (SIBDQ) was used to evaluate the quality of life. This questionnaire consists of 10 questions concerning the symptoms of illness, and the social and emotional sphere of the UC patient [10]. To assess satisfaction with the treatment a 10-point visual analogue scale (VAS) was used [11].
The data of the whole group were analysed and compared between 3 cohorts of patients as follows:
– Budesonide MMX® added to the 5-ASA drug 14 days or more after increase/optimization of 5-ASA dose for treatment of current flare or without 5-ASA dose modification (cohort 1);
– Budesonide MMX® added to the 5-ASA drug within 14 days since increase/optimization of 5-ASA dose for treatment of current flare (cohort 2);
– Budesonide MMX® used in monotherapy for treatment of current flare (cohort 3).
Statistical analysis
In the Polish sub-group of the study, 181 patients were recruited, and 177 of them completed the study. Based on that, the analysis per protocol of 177 patients was performed. The main outcome for assessment was the clinical improvement described as the percentage of patients with a change in the UCDAI clinical sub-score ≥ 3 points. The analysis also covered the percentage of patients with partial resolution of symptoms SF = 0 or RB = 0. The clinical remission was defined as UCDAI clinical sub-score ≤ 1 point and full resolution of symptoms as SF = 0 and RB = 0. The analysis of the clinical response was performed by comparing the results of descriptive statistics for the difference in outcome at the baseline and at the end of treatment. The patient’s quality of life was assessed according to the SIBDQ, comparing the results at the baseline and at the end. Satisfaction with treatment by VAS was evaluated at the end of treatment. The scale of clinical response and the impact on the quality of life of patients were compared in the whole group and in individual cohorts of patients. Descriptive statistics for category variables are presented as numbers and percentages, and for numerical variables as averages with standard deviation or median with quartiles. Comparisons of changes in the examined parameters of clinical evaluation and quality of life were made using Wilcoxon’s paired test. Comparisons between cohorts were carried out using χ2 tests for category variables and Kruskal-Wallis tests for continuous and orderly variables. The calculations were carried out using the statistical program R 3.4.0 (R Core Team [2017]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
Results
Study population
In the study population of 181 patients, women constituted 45.9%. The average age for the whole group was 39.9 years, and among them 2.8% were identified as smokers. In terms of the extension of inflammation, 55.8% of patients had left-sided colitis. In the whole group intolerance to 5-ASA and steroids was found equally in 3.9% of patients. There were no significant statistical differences between the cohorts. Baseline characteristics are summarized in Table I.
Table I
Baseline characteristics of the study population n (%)
In the past, during the last induction treatment, among 75 patients on oral steroid therapy, 25 of them reported adverse reactions to steroids. It occurred in 33.3% of patients in this group and in 13.8% of the entire study population. The most frequently reported symptoms were mood swings, sleep changes, moon face, and fluid retention. These symptoms occurred in 17 patients. Due to the low number of individual events, the statistical significance of differences between cohorts was not tested.
Clinical response in the whole study population
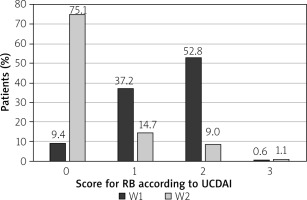
At the end of budesonide MMX® treatment at the 2nd visit (W2), 99 patients reported normal stool frequency (SF) = 0 (55.9%), whereas no rectal bleeding (RB) = 0 was reported by 133 (75.1%) patients. The changes in the percentage of patients with improved SF or RB were statistically significant (p < 0.001) (Figures 1 and 2).
Clinical improvement in the UCDAI clinical sub-score ≥ 3 points was reported in 113 (63.8%) patients. The clinical remission defined as UCDAI clinical sub-score ≤ 1 point was observed in 99 (55.9%) patients. Full resolution of symptoms SF = 0 and RB = 0 was reported in 93 (52.5%) patients. The differences in each category were statistically significant (p < 0.001) (Table II).
Table II
Clinical response in the whole study population
Clinical response in cohorts
The highest percentage of patients with SF = 0 or RB = 0 points was in the 2nd cohort, at 59.3% and 77.8%, respectively. There were no statistically significant differences between the cohorts in that category (Table III).
Table III
Changes in scores for SF and RB between visits: 1st (W1) and 2nd (W2)
The clinical improvement, clinical remission, and full resolution of symptoms in the highest percentage of patients was recorded in cohort 2 and was, respectively, 74.1%, 70.4%, and 55.6%. No statistical differences between cohorts were observed (Table IV).
Table IV
Clinical response in cohorts
Quality of life and satisfaction with treatment in the whole study population
The quality of life was assessed by the SIBDQ. In the whole study population, the quality of life was improved after the treatment. The outcome from the SIBDQ score increased from 40 to 56 points on average. The change was statistically significant (p < 0.001). Satisfaction with the treatment was assessed by 10-point VAS. At the end of induction therapy with the budesonide MMX® the mean value in the VAS for the whole study population was 7.79 ±2.89. Satisfaction with the treatment at the 10-point level was rated by 74 (41.8) patients. In total, 129 (72.9%) patients assessed satisfaction with treatment at ≥ 8 points.
Quality of life and satisfaction with treatment in the cohorts
In each of the analysed cohorts there was a statistically significant change in the SIBDQ score between the 1st and 2nd visit. There was no statistically significant difference between the cohorts in the change of SIBDQ score between the 1st and 2nd visit. In the evaluation of the treatment satisfaction by VAS scoring, the highest percentage of patients who evaluated budesonide MMX® treatment at 10 points was recorded in cohort 2. In all cohorts the percentage values were comparable, and therefore the statistical significance of differences between cohorts was not tested.
Safety
In 4 patients, the observation was not completed according to the protocol. Three of them did not attend the 2nd visit due to unknown reasons. The 4th patient was reported with an adverse event, which resulted in discontinuation of therapy and exclusion from the study. The event was reported by the investigator in accordance with the Polish legal system.
Discussion
Currently, budesonide MMX® is approved for the induction of remission in adult patients with mild-to-moderate UC in whom 5-ASA treatment is not sufficient [6]. Based on registration trials (CORE I and II), the efficacy of budesonide MMX® in monotherapy was proven. In a phase IIIB trial (CONTRIBUTE) the efficacy of budesonide MMX® was demonstrated in add-on therapy to 5-ASA. This subgroup study assessed the results of the 181 Polish patients participating in the European clinical trial CORE Practice: 177 patients were analysed per protocol. The clinical efficacy, quality of life, and satisfaction with the therapy were evaluated.
After 8 weeks of treatment with budesonide MMX®, 63.8% of patients achieved clinical improvement in the UCDAI clinical sub-score ≥ 3. In the pooled analysis of CORE I and II studies, the percentage of patients with improvement ≥ 3 points was 37.5% [12] and in the CONTRIBUTE trial 47% [5]. In the Polish population, the reduction by 3 or more points concerned only the clinical part of the UCDAI scale, so it is not possible to directly compare these results. However, in the Polish population of the CORE Practice and in the CONTRIBUTE trial, the percentage of patients with improvement in the UCDAI clinical sub-score ≥ 3 points were higher than in the CORE I and II trials. Budesonide MMX® was used as monotherapy in both CORE I and II whereas in CONTRIBUTE and CORE Practice it was used as add-on therapy to 5-ASA.
In this study, clinical remission was defined as UCDAI clinical sub-score ≤ 1 point. In the whole study population, the percentage of patients with clinical remission was 55.9%. In the CORE I and II studies, the primary endpoint was also ≤ 1 but on the total UCDAI scale. In a pooled analysis of these studies, the percentage of patients with UCDAI ≤ 1 was 17.7% [12]. In the CONTRIBUTE study, 13% of patients achieved clinical and endoscopic remission defined the same as in the studies (CORE I and II). In 2019, the results of a retrospective study evaluating the use of budesonide MMX® in add-on therapy in mild-to-moderate UC were published by Maconi et al. [13]. In this study 50% of patients achieved clinical remission defined as Mayo clinical sub-score ≤ 1 point. The results from that study and CORE Practice, which evaluated daily clinical practice, are therefore similar.
Full resolution of symptoms (SF = 0 and RB = 0 points) occurred in 52.5% of patients in the whole study population. In the pooled analysis of CORE I and II studies this percentage was 26.3% [12], and in the CONTRIBUTE study it was 24.3% [5]. In individual cohorts of the Polish population, the percentage of patients with full resolution of symptoms was 51.6%, 55.6%, and 54.5% for cohorts 1, 2, and 3, respectively. These results were comparable regardless of whether budesonide MMX® was used in add-on therapy (cohort 1 and 2) or in monotherapy (cohort 3). According to the ECCO guidelines, stool frequency and rectal bleeding are highly appropriate measures of the therapy evaluation [14, 15].
When evaluating the outcome of Polish patients in the CORE Practice study, it is important to consider their characteristics relative to patients in the CONTRIBUTE trial. The percentage of patients with mild disease was higher than in the CONTRIBUTE trial: respectively, 57.8% vs. 18.3% [5]. This is similar to the Maconi publication from 2019, in which clinical remission of 78% of patients was observed among those with mild UC [13]. In the Polish population of the CORE Practice study, most patients had left-sided colitis (55.8%), while in the CONTRIBUTE study this percentage was 36.5% [5]. In the Maconi group, the highest percentage of patients with clinical remission (75%) was among those with left-sided colitis or proctitis.
Patient’s quality of life in the Core Practice study was assessed by the SIBDQ. The increase of points between the 1st (W1) and the 2nd visit (W2) reflects the improvement in the quality of life. The average SIBDQ score in the whole population study at baseline was 40 points, and it was 56 points at the end of budesonide MMX® treatment. Patients are assumed to respond positively to treatment in terms of quality of life when a change in SIBDQ value is obtained from less than 49 points to more than 51 points [16].
The patients’ satisfaction with the therapy was assessed by visual analogue scale (VAS) at the end of the 8th week of budesonide MMX® treatment. A score of 1 means that the current treatment is completely unsatisfactory, and a score of 10 means full satisfaction with the treatment. In the observed study population, the average VAS score was 7.8 points, and over 40% of patients assessed their satisfaction at 10 points.
The adverse events in the Polish sub-group of the CORE Practice study occurred in a single patient, which gives a percentage of 0.6%. In the whole study population 38.4% of patients experienced a corticosteroid adverse event during the previous cycles of systemic steroid administration. It is estimated in the literature that more than 50% of patients treated with systemic steroids experience more than one side effect caused by these drugs [17]. In a pooled analysis of CORE I and II studies and in the CONTRIBUTE trial, the proportion of patients with glucocorticoid-related adverse events was 9% [5, 12]. In 2016, the results of a Czech observational study with budesonide MMX® used in 81 patients with mild-to-moderate UC were published [18]. In this group, 16% of patients had an adverse event during 8 weeks of observation. The low percentage of patients reporting adverse events after the use of budesonide MMX® was confirmed by the results of a meta-analysis comparing the safety of systemic and topical steroids. The meta-analysis covered the results of 31 clinical trials involving 5689 patients with IBD. The use of budesonide was statistically significantly less likely to cause adverse events than treatment with prednisone, or beclomethasone [19]. In another recently published meta-analysis, the risk of adverse events with budesonide MMX® was determined at the placebo level [20].
The limitation of the study is the lack of objective measures of inflammation to assess clinical efficacy such as endoscopy and/or faecal calprotectin test. Endoscopy or calprotectin measurement was optional because this was an observational study. Few patients underwent endoscopies and faecal calprotectin tests within the study.
The data from the Polish sub-group of the real-life study CORE Practice confirm the clinical efficacy of Budesonide MMX® 9 mg in the majority of patients with active mild-to- moderate UC. Budesonide MMX® was safe and well tolerated. Therapy was satisfactory for the patients and showed a beneficial effect on the patients’ quality of live.
In 2015, the guidelines of the Working Group of the Polish Society of Gastroenterology concerning the management of patients with Crohn’s disease and ulcerative colitis were updated. It is recommended that Budesonide MMX® be used at a dose of 9 mg/day for induction therapy of mild-to-moderate UC if no remission is obtained after 5-ASA or if intolerance of 5-ASA preparations is observed. The drug should be recommended by indication for steroid therapy, but before systemic corticosteroids [21].