Introduction
Pancreaticoduodenectomy (Kausch-Whipple procedure) is the standard operation for lesions located in the periampullary area. The mortality rate of the procedure has decreased to less than 5% in high-volume centres; however, the morbidity rate remains significantly high (40–50%). One of the major post operative complications (14–61%) after pancreaticoduodenectomy or pylorus-preserving pancreaticoduodenectomy is delayed gastric emptying (DGE) [1–3]. According to ISGPS, DGE is the requirement of a nasogastric tube after post-op day 3 or failure to resume an oral diet by post-op day 7. Several factors have been related to DGE, such as vagal denervation, loss of neural connection between the stomach and small intestine, decreased motility due to duodenal resection, technical aspects of gastroenteroanastomosis, pancreaticojejunostomy failure, and retrogastric collection [3–8].
Perineural invasion is defined as the presence of cancer cells within the epineural, perineural, and endoneural spaces around the nerves and is considered a negative prognostic factor associated with increased local recurrence rates. Perineural invasion occurs in approximately 70–90% of cases of pancreatic cancer [9–14] and has been shown to be related with cancer-associated pain and hyperglycaemia [14–16]. Midkine and syndekan-3 are 2 factors expressed in pancreatic cancer, and they are associated with perineural invasion [11]. The presence of perineural infiltrations is considered a negative prognostic factor, associated with poor overall survival and increased recurrence rates [16–20].
Aim
The primary aim of this study is to assess if there is any correlation between the existence of perineural invasion and postoperative DGE, and the secondary aim is to assess the impact of perineural invasion, DGE, and several clinical factors on overall survival.
Material and methods
Patients
In this retrospective observational double-centre study, 142 patients underwent pancreaticoduodenectomy for periampullary tumours in Evaggelismos General Hospital of Athens and University Hospital of Patras from 2013 to 2019 for curative reasons. Follow-up from the outpatient department was missing for 8 cases, and 8 cases were excluded because biopsy showed chronic pancreatitis, and another 3 cases were excluded because the biopsy showed benign serous cystadenoma. Thus, 123 cases were enrolled in the study, and more specifically 113 patients underwent classic Kausch-Whipple procedure, 5 patients underwent pylorus-preserving pancreaticoduodenectomy, and 5 patients underwent total pancreatectomy. They were 73 males and 50 females ranging from 31 to 90 years old (mean ± SD = 68 ±10.9). Patients were followed up until 1/1/2020, which was the cut-off date for all information gathered. Participating centres were responsible for completion of local approvals before the start of data collection.
Statistical analysis
Associations between categorical attributes such as DGE and perineural invasion were examined using Pearson’s χ2 test of independence. For the variable age, the Mann-Whitney non-parametric test was used after checking normality plots. DGE, perineural invasion, and several clinical parameters of potential prognostic value, such as age, gender, existence of postoperative fistula, and haemorrhage, were analysed for their effect on overall survival using Cox regression univariate and multivariate analyses. Moreover, analyses of overall survival were accomplished with Kaplan-Meier plots, where the differences in survivals between the groups were compared using the log-rank test. In all statistical tests, the significance level was defined as p < 0.05. All statistical analyses were implemented using SPSS statistical tool (SPSS Statistics 24 Chicago, IL, USA).
Results
In the study, 123 patients were enrolled, who underwent pancreaticoduodenectomy. The pathological specimen showed perineural infiltration in 98 (79%) cases whereas 25 cases had no perineural invasion (21%). At clinical follow-up, 39 (32%) patients developed delayed gastric emptying, in contrast to 84 (68%) patients who did not present symptoms of DGE. Postoperative fistula was diagnosed in 47 (38%) cases, which was grade A in 37 cases, grade B in 6 cases, and grade C in 4 cases. Postoperative haemorrhage was found in 19 (15%) cases and was treated conservatively in 10 patients, with percutaneous transarterial embolization in 4 cases, and 5 patients were operated urgently. The source of bleeding was the GDA stump in most cases, retropancreatic vessels, and in 1 case the point of haemorrhage was a splenic artery pseudoaneurysm. The days of hospitalization ranged from 8 days to 115 days (mean SD = 36 ±21.1). Six patients died postoperatively (mortality = 6%), and the mean overall survival in months was 21.7 ±18.6 (Table I).
Table I
Clinical features of all 123 patients included in the study
Table II shows the statistical association between perineural infiltrations and other clinical factors with DGE. As is shown, the presence of perineural infiltrations is statistically correlated in a positive way with development of DGE (p = 0.01), which means that the presence of perineural infiltrations increases the possibility of clinical DGE (odds ratio = 4.2, 95% CI: 1.191–15.22). Thus, perineural infiltrations could be used as a clinical predisposing factor for the DGE effect, in addition to being an important negative prognostic factor for survival and recurrence. Moreover, there is a positive association between gender and DGE, showing that males may be more susceptible to development of DGE (p = 0.001, odds ratio = 2). The statistical analysis showed no correlation between the presence of postoperative fistula and DGE (p = 0.969); however, there was a positive statistical correlation between the grade of postoperative fistula and DGE (p < 0.01), which was anticipated because grade B and C pancreatic fistulas worsen the patient’s clinical condition, increasing the possibility for symptoms of DGE (p < 0.01). In addition to this, postoperative haemorrhage is statistically associated with the presence of DGE (p = 0.03, odds ratio = 2.8). There was no correlation between age and DGE (p = 0.845). Further analyses were performed to confirm possible statistical correlation between perineural infiltrations and other clinical factors, except DGE, as is shown in Table III. However, no statistical correlation was found (p = 0.272 for age, p = 0.164 for gender, p = 0.505 for postoperative fistula, p = 0.535 for postoperative fistula grade, and p = 0.932 for postoperative haemorrhage) (Table III).
Table II
Association between DGE and clinical features in all 123 cases included in the study
Table III
Association between perineural infiltrations and other clinical features in all 123 cases included in the study
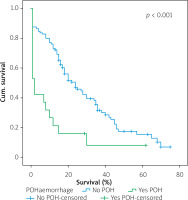
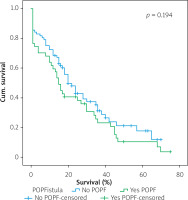
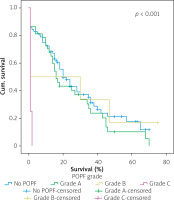
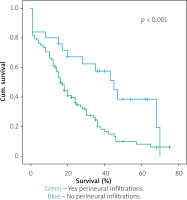
Table IV shows the results of univariate cox regression survival analysis. Thus, increased overall survival was found to be significantly associated with age (p = 0.018), which means that younger people have increased survival, with absence of perineural infiltrations (p = 0.005), and with postoperative fistula grade. In general, increased overall survival is associated with absence of postoperative fistula; however, the grade is an important factor, and the highest-grade fistula has the worst prognosis and overall survival (p = 0.001 for grade A, p = 0.03 for grade B, and p = 0.005 for grade C). Furthermore, the absence of postoperative haemorrhage is correlated with increased survival (p < 0.01). There was no statistically significant association between survival and clinical factors, such as presence of DGE (p = 0.574), postoperative fistula (p = 0.194), and gender (p = 0.768).
Table IV
Univariate cox regression survival analysis and log-rank test survival analysis of all 123 cases included in the study
Table V shows the results of multivariate Cox regression analysis. The absence of perineural infiltrations (p = 0.006), young age (p = 0.014), and the absence of postoperative haemorrhage (p < 0.001) are independent prognostic factors that are associated with increased overall survival and good prognosis. Conversely, no statistical correlation was found in multivariate analysis between gender (p = 0.730), presence of postoperative fistula (p = 0.09), postoperative fistula grade (p = 0.118), presence of DGE (p = 0.352), and overall survival.
Table V
Multivariate Cox regression survival analysis of all 123 cases included in the study
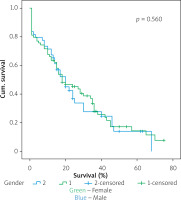
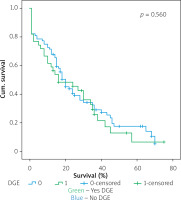
Figures 1–7 display the results of Kaplan-Meier survival analyses. The log-rank test shows that increased overall survival is statistically associated with younger age (p = 0.028), low grade of postoperative fistula (p < 0.001), absence of postoperative haemorrhage (p < 0.001), and absence of perineural infiltrations (p < 0.001), whereas no significant statistical correlation was found between overall survival and gender (p = 0.560), presence of postoperative fistula (p = 0.194), and DGE (p = 0.560) in the log-rank test.
Discussion
Pancreatic cancer is the fourth leading cause of cancer-related death in Western countries, and it is known for its aggressiveness, resulting in a 5-year survival rate below 10% [1]. Pancreaticoduodenectomy with adjuvant chemotherapy is the only curative pathway; however, significant complications make the therapeutic team’s work difficult. One of the most common complications is DGE, which causes significant discomfort, elongated hospital stay, frequent readmissions, and increased hospital costs [1]. The aetiology is unknown, but several factors, such as loss of neural connection between the stomach and intestine, vagal denervation, functional obstruction due to stomach dysrhythmia, decreased levels of motilin due to duodenal resection, technical matters like gastroenteroanastomosis angulation, retrogastric collection, and the presence of pancreaticojejunostomy failure with or without pancreatic fistula have been described as contributing causes of DGE [1–3]. Hashimoto et al. performed a multivariate analysis, which showed that the presence of pancreaticojejunostomy failure and the absence of surgical microscope are 2 independent factors responsible for DGE [2], whereas Bassi et al. found that postoperative pancreatic fistula is an independent factor for DGE [7]. In this study, a statistical correlation was found between the presence of perineural infiltrations and DGE (p = 0.01, odds ratio = 4.2, 95% CI: 1.191–15.22). This is the first study that proves that the presence of perineural infiltrations increases the possibility of DGE in the postoperative period. The possible explanations for this case are, first of all, the extensive nerve dissection in the hepatoduodenal ligament and the hepatic artery planes, so as to perform lymph node clearance for satisfactory oncological outcome. It is well known that the pancreas is a highly enervated organ. The coeliac plexus lies between the pancreas and the superior mesenteric artery innervating the head of the pancreas, whereas the splenic plexus innervates the tail and body of the pancreas. Thus, the destruction of adjacent nerves during surgical dissection of the pancreas may lead to denervation of the stomach and clinical DGE. Second, perineural invasion is a complex interaction between tumour cells, nerve cells, and stromal cells via paracrine and autocrine mechanisms, involving several factors like neurotrophic GFs, CSF-1, GDNF glial-derived neurotrophic factors, and other inflammation mediators, which alter the inherent neural function and electrophysiological patterns of neural message transmission. Thus, the nerves that remain after the pancreatic dissection may have an abnormal function, which has an impact on gastric motility. Several studies in mice and in vitro studies have proven the above-mentioned microenvironmental changes and neurotrophic factor alterations [14–22]. Finally, it must be taken into account that after pancreaticoduodenectomy, there might be sites of nerves with remaining perineural invasion and nerves being displaced by local oedema or fluid collections that have impaired function, which may lead to DGE.
Furthermore, postoperative DGE was found to be statistically correlated with gender (p = 0.001), postoperative fistula grade (p < 0.01), and postoperative haemorrhage (p < 0.01). That means that males present DGE more often than do females, which has not been described in the literature before. Postoperative pancreatic fistula formation is a dreaded complication that contributes to DGE, postoperative haemorrhage, sepsis, and intra-abdominal collections. Obviously, the worse the pancreatic fistula grade, the higher the probability of DGE, because the clinical condition of the patient becomes critical with multiple organ dysfunction, including the stomach. Postoperative haemorrhage is usually related with pancreaticojejunostomy failure and gastroduodenal artery stump wall erosion, which leads to haemorrhage. The patient once more is in critical condition with the occurrence of DGE being very probable. However, in the study there is a bias concerning the number of grade B and C pancreatic fistulas (n = 6 and n = 4, respectively), which is significantly low, in contrast to grade A (n = 37) pancreatic fistula, which refers to a more stable and safe clinical condition. Malleo et al. in their study found that DGE was associated with the grade of the pancreatic fistula, as in this study, but there was no association with age or postoperative haemorrhage [7].
Perineural invasion is a poor prognostic factor that is associated with increased recurrence rates and decreased overall survival in pancreatic cancer, as well as gastric cancer, cholangial cancer, prostate cancer, and lung cancer [13–20]. In this study we did not find any statistical association between perineural invasion and other clinical factors apart from DGE, in accordance with Yang et al., who had similar results. However, Yang et al. [13] did not take into account factors like postoperative fistula and haemorrhage. On the other hand, they analysed factors like lymph node metastases, tumour differentiation, pancreatitis, and Ca19-9 levels and found that there is statistical association of these factors with perineural invasion [13].
Univariate Cox regression analysis showed that increased overall survival is associated with age (Table IV), meaning that older people have lower overall survival, with the presence of perineural infiltrations, which is a poor prognostic factor, with the postoperative pancreatic fistula grade (grade C having the worst prognosis and grade A the best prognosis), and finally with the presence of haemorrhage. That means that the occurrence of postoperative haemorrhage deteriorates the overall survival. To increase the sensitivity of the results, a log-rank test with Kaplan-Meier diagrams extracted was performed, which showed the same results. The only factors that were not statistically correlated with overall survival were gender, DGE, and presence of pancreatic fistula. Multivariate analysis showed that age, perineural invasion, and postoperative haemorrhage are independent prognostic factors. The presence of pancreatic fistula postoperatively had only limited statistical significance (p = 0.09). The fact that both Cox regression survival analysis and log-rank test have exactly the same results increases the credibility of them. Most of the studies in the bibliography agree that perineural invasion is a significant negative prognostic factor, which is associated especially with disease recurrence [16–22]. A weak point of this study is that it analysed the overall survival time and not the disease-free survival time. Han et al. [9] and Yao et al. [10] point out that perineural invasion is an independent prognostic factor along with lymph vascular invasion, but perineural invasion may occur in the early stages, even without lymphovascular invasion and in tumours < 2 cm. Thus, perineural invasion occurs quite often and is a significant poor prognostic factor [9, 10].
Conclusions
This is the first study that proves a statistical association between DGE and perineural infiltrations found in pathological reviews after surgery, but more studies are needed to prove the current dataset. Moreover, perineural invasion is an important independent negative prognostic factor, which may be used in clinical practice to make decisions on the therapeutic plan after the operation.