Introduction
Menopause is the cessation of the ovarian follicular activity followed by the termination of the menstrual cycle [1]. The age of menopause varies in different societies, but on average it occurs between 48 and 52 years old [2, 3]. The incidence and severity of menopausal symptoms vary between women and are affected by various factors such as genetics, environment, anthropometrics, lifestyle, and race. Menopause can cause a wide range of symptoms in different systems due to the decrease in estrogen during this period [4]. Vasomotor disorders [4], mood disorders [5], and urogenital atrophy [6] are among possible complications. Early menopause is also associated with an increased risk of osteoporosis [7], coronary artery disease, stroke, and death [8]. These factors can affect women’s health and quality of life. However, one of the most important symptoms of these patients is the symptoms related to metabolic disorders. Menopause can lead to an increase in total body fat and the distribution of body fat from the periphery to the trunk, resulting in visceral obesity [9, 10]. Abdominal obesity and estrogen reduction during menopause are associated with adverse metabolic changes such as insulin resistance, tendency to develop type 2 diabetes, and dyslipidemia [9, 11].
Due to the increase in the prevalence and occurrence of type 2 diabetes in recent decades, paying attention to possible risk factors of this disease has become very important [12]. The rate of disability- adjusted life years (DALYs) for women with T2DM increased by 34.2% from 2007 to 2017 [13]. Moreover, at least one of the T2DM risk factors, including hypertension (HTN), dyslipidemia, central obesity, and increased fasting glucose level, occurs in 50-80% of menopausal women [14]. Therefore, identifying potential risk factors for T2DM, such as menopause, is critical for early detection, prevention, and management. However, few studies have investigated the effect of menopause on the probability of type 2 diabetes. The results of these studies are conflicting. For example, some studies point to an increase in the probability of type 2 diabetes with a decrease in the age of menopause [15, 16]. However, other studies do no support such a relationship [17, 18]. Therefore, in this systematic review and meta-analysis, we will analyze the available studies on the relationship between the age of menopause and the risk of type 2 diabetes.
Methods
This systematic review and meta-analysis aimed to investigate the association of menopausal age and T2DM. This study adhered to the principles of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA Checklist). The protocol of this study is available at the Open Science Framework (OSF) website (OSF: https://osf.io/26p38/).
Search strategy
We searched PubMed, Scopus, and Google Scholar databases to find all studies associated with menopause and type 2 diabetes up to January 24, 2024. We also manually checked the reference lists of relevant studies to ensure that all studies were found. The search strategy of this systematic review and meta-analysis is available in Table 1.
Table 1
Search strategy for current systematic review and meta-analysis
Study selection
Studies were reviewed for compliance with the inclusion criteria by two authors (A.A, R.HZ). Inclusion criteria included: 1) study design, prospective cohort, cross-sectional studies, case-control, and randomized controlled trials (RCTs); 2) participants were postmenopausal women (women who experienced natural or surgical menopause) without a history of type 2 diabetes at baseline; 3) reporting age of menopause; 4) type 2 diabetes diagnosis is confirmed through clinical or laboratory criteria; 5) studies should report sufficient data to calculate odds ratios (ORs) and 95% confidence intervals (95% CI) for the association between age at menopause and type 2 diabetes mellitus (T2DM).
Data extraction and quality assessment
Two separate reviewers (FAM and QB) extracted the necessary information based on the standard form. Information including author name, year, type of the study, sample size, and number of T2DM in each category of menopausal age was extracted from studies.
The quality assessment of the studies was performed by two reviewers (QB and AA) separately, and any disagreement was solved by discussion.
Statistical analysis
Data about the relationship between menopause and T2DM were extracted from included studies. Due to different outcome reporting, the age categories were unified into several groups: A) menopausal age > 45 vs. menopausal age < 45; B) menopausal age > 50 vs. menopausal age < 45, and C) menopausal age > 55 vs. menopausal age < 45. Moreover, surgical menopause (including oophorectomy and hysterectomy) vs. natural menopause constituted a separate category. Then, a meta-analysis was performed by calculating the ORs and 95% CIs using the random effect model and the DerSimonian-Laird method. Additionally, a forest plot was prepared to show the pooled effect size. We used leave-one-out analysis to ensure that the result did not have a great impact on any single study. Heterogeneity between included studies was evaluated using Cochran’s Q test and I2 statistic, and I2 > 50% was considered to indicate heterogeneity. Subgroup analyses were planned to explore potential sources of heterogeneity, including study design (cohort vs. cross-sectional), menopausal age categories (early vs. late), and geographic location. Interaction tests were performed to evaluate differences between subgroups.
Publication bias was evaluated through funnel plot, Egger’s test, and trim and fill analysis. All statistical analyses were performed using STATA version 17, and p-values < 0.05 were considered statistically significant.
Results
Study selection
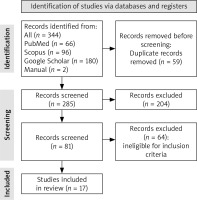
Through a comprehensive search of three datasets, we found 345 relevant studies. We obtained three additional studies from the references list of previous meta-analyses. After removing duplicate studies, 286 studies remained. Then, 204 irrelevant studies were excluded through title and abstract screening. The full text of the remaining studies was evaluated, and 65 records were removed due to ineligibility with our inclusion criteria. Finally, 17 studies were included for systematic review and meta-analysis. The study selection procedure is shown in Figure 1.
Study characteristics
The selected studies were published between 2013 and 2022 and included a total of 421 801 menopausal woman participants, of whom 37 656 had T2DM. Geographical diversity was observed, with studies conducted in China [19–26], the USA [27, 28], Europe [16], Japan [29, 30], The Netherlands [31], France [32], Egypt [33], and ten different countries [34]. The studies employed different study designs, including cohort [16, 19–22, 27, 29–32, 34] and cross-sectional [23–25, 28, 33] studies (Fig. 2). The characteristic features of included studies are shown in Table 2.
Fig. 2
World map showing the distribution of diabetic menopausal women
This figure was made using free Canva elements.
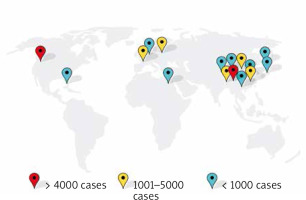
Table 2
Characteristics of included studies
| Author, year | Country | Type of study | Sample size | Number of cases | Type of menopause |
|---|---|---|---|---|---|
| Brand 2013 [16] | Europe | Case-cohort | 7864 | 3691 | Not mentioned |
| Heianza 2013 [29] | Japan | Cohort | 4416 | 170 | Natural/Surgical |
| Jiang 2019 [26] | China | Longitudinal | 2689 | 292 | Natural |
| LeBlanc [27] | USA | Cohort | 124379 | 11262 | Natural/Surgical |
| Lee 2013 [30] | Japan | Cohort | 4318 | 140 | Natural |
| Li 2020 [23] | China | Cross-sectional | 5653 | 990 | Not mentioned |
| Muka 2017 [31] | The Netherlands | Cohort | 3639 | 384 | Natural |
| Pandeya 2018 [34] | 10 different countries | Cohort | 126721 | 4073 | Natural |
| Qiu 2013 [24] | China | Cross-sectional | 2940 | 373 | Not mentioned |
| Seddek 2016 [33] | Egypt | Cross-sectional | 357 | 122 | Not mentioned |
| Shen 2017 [19] | China | Cohort | 16299 | 2811 | Natural |
| Tatulashvili 2020 [32] | France | Cohort | 83799 | 4806 | Not mentioned |
| Wang 2022 [20] | China | Cohort | 141789 | 7740 | Natural |
| Xing 2022 [28] | The USA | Cross-sectional | 4963 | 796 | Natural/Surgical |
| Yang 2016 [21] | China | Cohort | 5063 | 640 | Natural |
| Yu 2022 [25] | China | Cross-sectional | 14353 | 2521 | Natural/Surgical |
| Zhang 2020 [22] | China | cohort | 15406 | 1952 | Not mentioned |
Menopausal age was collected through self-reporting or medical documents in the natural and surgical menopause process, respectively. Additionally, T2DM was diagnosed through laboratory examination. Different studies reported their data in various categories. Therefore, we unified participant categories into three groups: A) menopausal age < 45 vs. menopausal age > 45; B) menopausal age < 45 vs. menopausal age > 50; and C) menopausal age < 45 vs. menopausal age > 55.
Menopausal age < 45 vs. menopausal age > 45
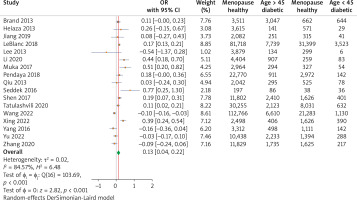
The combined analysis of 17 studies demonstrated a significant association between menopausal age and risk of T2DM. The calculated OR demonstrated that women with menopausal age > 45 showed a significant relation with T2DM development with an OR of 0.13 (95% CI: (0.04, 0.22), p < 0.01, indicating a 13% protective effect compared to menopausal age < 45. The studies demonstrated high heterogenicity, with an I2 of 84.57. The forest plot diagrams are presented in Figure 3.
Menopausal age < 45 vs. menopausal age > 50
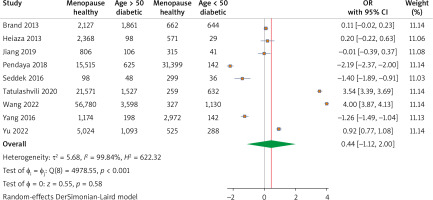
A total of nine studies reported overall diabetic and non-diabetic cases with menopausal age greater than 50 years old. The pooled analysis revealed a substantial effect of menopausal age on T2DM development with an OR of 0.44 (95% CI: –1.12, 2.00), p > 0.05, I2 = 99.84% (Fig. 4).
Menopausal age < 45 vs. menopausal age > 55
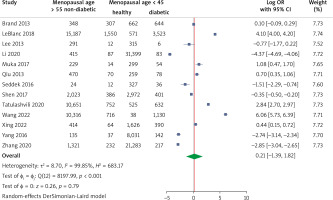
The pooled analysis of 13 studies showed no significant effect of menopausal age on the risk of T2DM with an OR of 0.21 (95% CI: –1.39, 1.82), p > 0.05. Also, it showed strong heterogenicity, with an I2 of 99.85 (Fig. 5).
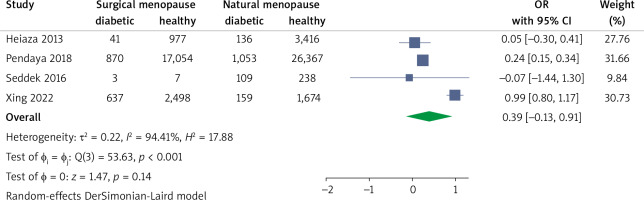
Surgical menopause vs. natural menopause
A total of 4 studies reported diabetes in surgical and natural menopause women separately. The surgical status showed an OR of 0.39 (95%CI: –0.13, 0.91), which was not statistically significant (Fig. 6).
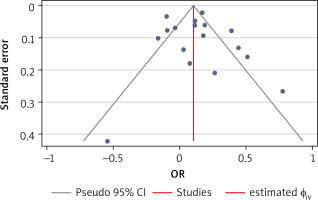
Publication bias
We assessed publication bias using Egger’s test, funnel plot, and trim-and-fill analysis. The funnel plot (Fig. 7) exhibited a symmetrical pattern, indicating no publication bias. This result was supported by Egger’s test (p > 0.05) and trim-and-fill analysis (no imputed studies).
Discussion
This present meta-analysis unified the information of 17 studies with 421 801 menopausal women and 37 656 T2DM cases to evaluate the complex association between age at menopause and risk of developing T2DM. Merging data showed the multifactorial nature of this relationship, which needs further investigation. The syncretistic analysis showed that earlier menopausal age is significantly related to an increased risk of T2DM, while later menopausal age is associated with decreased risk of T2DM. This finding plays a critical role in our understanding of the association of hormonal alteration during menopause with the risk of metabolic disorders.
Our meta-analysis revealed a notable effect of menopausal age on the risk of T2DM development. Women with menopausal age higher than 45 have a 13% lower risk of developing T2DM. This effect increased to 44% for menopausal age more than 50. However, by increasing the age of menopause, this upward trend did not continue. Also, the probability of not developing diabetes was reduced to 21%, which still was higher than the menopausal age of > 45.
The correlation observed between the earlier onset of menopause (whether natural or surgical) and the risk of developing T2DM aligns with the findings of recent investigations. These findings suggest that a shorter period of exposure to endogenous estrogen may play a role in T2DM pathogenesis [35]. This relation corresponds with the recognized effect of ovarian hormones, especially estrogen, on the functioning of pancreatic beta cells and the maintenance of glucose homeostasis [36].
There are many disagreements among researchers that are worth investigating. Some studies did not report a significant relationship after adjusting confounding variables such as BMI, blood pressure, etc. [17, 18]. However, there are other studies that, similar to the results of our meta-analysis, have observed a positive relationship between early menopause and the risk of type 2 diabetes [15, 16]. This variation in the observed results indicates the complexity of this relationship and suggests that other factors may influence the observed associations.
One of the reasons for inconsistencies in the results may be the lack of a standard definition of surgical menopause in different studies. Surgical menopause includes various methods, including oophorectomy and hysterectomy [16], in which hormonal profiles can be significantly different [37, 38]. This variation in definitions of surgical menopause and differences in hormone levels can mask the true nature of the relationship between age at menopause and the risk of type 2 diabetes.
In addition, the menopause process is characterized by complex hormonal alterations. At first, there is an initial increase in estradiol secretion followed by a gradual decline, while blood testosterone levels remain relatively constant [39]. Also, the increase in androgenicity during menopause can be associated with a higher incidence of T2DM [40, 41]. These changes in women’s hormonal profiles can affect metabolic processes and potentially contribute to diabetes risk.
In addition, other changes occur in the human body with menopause. This includes an increase in fat mass, an increase in abdominal fat, and a decrease in body fat-free mass, resulting in metabolic changes, all of which can be risk factors for the development of T2DM [42]. Regular physical activity and lifestyle modification can act as factors that influence metabolic changes, weight gain, and alteration in body fat distribution after menopause [43]. For this reason, early menopausal women have more time exposed to these adverse changes, and the risk of T2DM is higher for them than for later menopause.
Our findings also raise interesting questions about the role of reproductive lifespan as a marker of lifetime estrogen exposure. Some experimental studies have shown that estrogen may have protective effects on insulin secretion, glucose metabolism, and diabetes risk [44, 45]. Therefore, assessment of reproductive lifespan, which accounts for the cumulative effect of estrogen exposure, may provide a more accurate understanding of the relationship between age at menopause and T2DM than age at menopause alone.
Another important consideration is the effect of postmenopausal obesity. Late menopause was associated with an increased risk of T2DM in non-obese subjects, while it was associated with a decreased risk in obese subjects [26]. Increased risk of diabetes after late menopause in non-obese women may be due to prolonged exposure to estradiol before menopause [46, 47]. This emphasizes the complex interplay between menopause, hormonal changes, and metabolic factors.
The effect of estrogen on glucose metabolism also appears to be multifaceted. Some evidence suggests a protective role of exogenous estrogen, particularly in glucose homeostasis [48]. Estrogen replacement in postmenopausal women causes lower incidence of T2DM [44, 45, 48]. Higher endogenous estrogen levels in postmenopausal women are associated with elevated glucose and insulin levels and an increased risk of diabetes [41, 47]. The complex relationship between estrogen and the risk of type 2 diabetes deserves further investigation.
Recent studies have shown that premature menopause is probably an indicator of premature aging. This is characterized by a decrease in the ability to repair and regenerate DNA. This happens both in the tissues of the reproductive system and in other tissues of the body. Thus, women who have a less efficient DNA repair system may be at risk of premature menopause and other chronic diseases, including diabetes [50–53].
The observed differences in the association between age at menopause and risk of T2DM across countries [32, 54] emphasize the need to consider diverse populations and their unique genetic and environmental backgrounds [35]. Social and environmental factors, such as education, smoking habits, and occupational status, can also influence age at menopause and potentially mediate the relationship with T2DM [25].
One of the complications of menopause is metabolic syndrome occurrence. This syndrome is associated with increased waist circumference, hyperglycemia, increased triglycerides, decreased HDL, and hypertension. These changes can increase the risk of diabetes in menopausal women. In addition, metabolic syndrome causes obesity by reducing metabolism and thus reducing calorie consumption. Also, this syndrome is associated with fat distribution in the abdominal area. All these factors contribute to the unique role of metabolic syndrome in the development of diabetes in postmenopausal women [55–58].
Furthermore, the etiology of T2DM is multifactorial, with genetic, lifestyle, and environmental factors all contributing [32]. These factors could vary by ethnicity and potentially influence the associations we observed. Diet can help determine BMI and body fat distribution. This effect is not only related to the amount of food consumed, but also the composition of food plays an important role. A diet containing large amounts of fat, red meat, and sweets can contribute to obesity and diabetes. Fruits and vegetables, by contrast, have a protective effect [59–62].
Due to the observed protective effect of higher menopause age on type 2 diabetes, women with menopause age below 45 years need more preventive measures than women with higher menopause age. As mentioned before, type 2 diabetes is a multifactorial disease, and one of the modifiable factors in the etiology of this disease is the diet of people.
In a study, Jin et al. investigated the effect of diet on the risk of type 2 diabetes in 11 000 postmenopausal women. They used an empirical dietary index for hyperinsulinemia (EDIH) and empirical dietary inflammatory pattern (EDIP) scores. The high level of these criteria indicated a hyper-insulinemic and hyper-proinflammatory diet. Finally, it was observed that the diet with a higher EDIH and EDIP score was associated with an increased risk of type 2 diabetes [63]. The EDIH and (EDIP) scores measure the protein C and inflammatory markers, respectively. These substances play a role in the etiology of several chronic diseases, including T2DM [64, 65].
Another study by Vitale et al. investigated the effect of micronutrients on metabolic parameters in postmenopausal women. In this study, the participants were treated with calcium, vitamin D, isoflavone, and insulin for 12 months versus placebo. Finally, it was observed that the amount of HDL in the case group was significantly higher compared to the placebo group [66]. The importance of increasing HDL in this study is shown by the observation that a lower HDL level is associated with an increased T2DM risk [67, 68]. Therefore, increasing the level of HDL serum through modifying the diet with calcium, vitamin D, and isoflavone can help prevent diabetes in postmenopausal people.
This meta-analysis displays several remarkable strengths. Primarily, we conducted a comprehensive and complete search to identify all relevant articles related to this topic and defined specific criteria to select the best research. Furthermore, during the data extraction process, we collected the most adjusted model data to minimize the potential confounding variables. Additionally, it is worth mentioning that the included studies contain investigations on both natural and surgical menopause, thereby enhancing the depth and extent of this meta-analysis.
Nonetheless, our study contains certain limitations. Included studies utilized self-reported data to determine menopausal age, which leads to concerns about potential recall bias. Nevertheless, prior investigations have demonstrated the reasonable reliability of this data collection method [69, 70]. Moreover, we performed leave-one-out analysis to ensure the reliability of our results, the outcome of which did not change significantly. This supports the robustness of our meta-analysis.
Conclusions
In conclusion, this present meta-analysis emphasizes the importance of the complex relationship between earlier menopausal age and the risk of T2DM development. It is necessary to investigate the confounding variables of this association, such as hormonal, genetic, and lifestyle factors. Future investigations must focus on the underlying mechanistic pathways. Understanding these mechanisms will help us with preventive strategies and interventions in post-menopausal women.