Introduction
Colorectal cancer is one of the most common malignant neoplasms and the fourth most common cause of death due to cancer worldwide. The incidence of colorectal cancer in an individual increases after the 5th decade of life. In 2020, approximately 1,148,515 new cases were diagnosed, and 576,858 deaths were recorded [1].
In general, colorectal cancer is on the third place in terms of incidence and second place in the category of causes of deaths from neoplastic diseases [1]. In Poland, in 2017, colorectal cancer incidence was fourth among men and fifth among women [2]. Despite the progress in diagnostics and therapy, a systematic increase in these statistics has been observed.
The etiopathogenesis of colorectal cancer consists of an improper diet, being overweight and obese, sedentary lifestyle, smoking, diabetes, excessive alcohol consumption, inflammatory bowel diseases, and acromegaly [2–4].
Oxidative stress is a condition in which there is an imbalance between the actions of reactive oxygen species (ROS) and the cell’s ability to neutralize and eliminate them [5–7]. The increase in the level of ROS corresponds to the increased production by cellular metabolism and the decreased rate of excretion. In the process of carcinogenesis, the increase in ROS leads to DNA damage, which translates into the formation of genetic mutations, but also impairs apoptosis and stimulates proliferation [5–11]. The factors predisposing to the intensification of oxidative stress include smoking, alcohol, stress, toxins and inflammatory processes related to metabolic diseases, as well as diet and lifestyle [6–13].
To assess oxidative stress, indicators such as total oxidant status (TOS), total antioxidant capacity (TAC), and oxidative stress index (OSI), can be used. TOS and TAC can be measured in serum or tissue, while OSI is a parameter derived from TOS and TAC values [6–16]. Furthermore free malondialdehyde (MDA) can be determined [17, 18]. This aldehyde is one of the products of lipid peroxidation; at the same time it is highly cytotoxic and has carcinogenic properties. Therefore, its serum level is used to assess the severity of oxidative stress. The TAC/MDA parameter can also be calculated to assess oxidative stress [17, 18].
Protein-caloric malnutrition is another phenomenon that occurs in cancer patients. It is estimated that this problem affects 20% to 80% of patients with gastrointestinal neoplasms [18–22]. To assess the nutritional status, various scales and markings are used including body mass index (BMI), unintentional weight loss, total protein and albumin levels, or the nutritional risk screening scale (NRS 2002).
Aim
Assessment of oxidative stress in patients with colorectal cancer and its severity depending on the nutritional status of patients.
Material and methods
The research was approved by the Bioethics Committee of the Medical University of Bialystok, Poland (permission number R-I-002/153/2017). After a thorough explanation of the purpose of the study and possible risks, qualified patients consented in writing to participate in the experiment. The study was conducted in accordance with the World Medical Association Declaration of Helsinki for ethical principles for medical research involving human subjects.
The study included 50 patients with colorectal cancer treated surgically at the 2nd Clinical Department of General, Gastroenterological and Oncological Surgery of the Medical University of Bialystok in 2017-2018. There were 19 women and 31 men in the study group. The mean age of the examined patients was 67 years. To prevent an additional increase in the level of oxidative stress, patients with diabetes, chronic renal failure, Parkinson’s disease, Alzheimer’s disease, and alcohol-dependent patients were excluded from the study group.
BMI was assessed on admission in all patients, and the NRS 2002 scale was filled in. Histopathological diagnosis was consistent with WHO standards. However, tumor advancement, lymph node metastases, distant metastases, as well as disease advancement were assessed based on the TNM UICC classification. Characteristics of the study group have been shown in Table I.
Table I
Characteristics of the study group
In the control group, selected according to sex and age to reciprocate the study group, samples were obtained from 40 healthy subjects attending follow-up visits at the Specialist Dental Clinic (Department of Restorative Dentistry) at the Medical University of Bialystok from January 2018 to January 2019. Only patients with normal results of complete blood count and biochemical blood tests (Na+, K+, creatinine, INR, ALT, AST) were admitted to the control group.
BMI was determined according to the formula: body weight/height in meters2, the result < 18.49 kg/m2 indicates underweight, 18.5–24.99 kg/m2 normal body weight, 25–29.99 kg/m2 overweight, while over 30 we are talking about obesity (obesity of I, II and III degree).
The values obtained by the patients were also assessed in accordance with the NRS 2002 scale. A score equal to or greater than 4 points on this scale may suggest malnutrition.
Blood collection
Fasting venous blood (10 ml) was collected from all patients on empty stomach and upon overnight rest. The S-Monovette® K3 EDTA blood collection system (Sarstedt, Germany) was used for this. Blood was centrifuged at 1500 x g for 10 min at +4°C (MPW 351, MPW Med. Instruments, Warsaw, Poland) and the top layer (plasma) was taken. To prevent sample oxidation, 0.5 M butylated hydroxytoluene (20 µl/2 ml plasma or serum) was added [18]. Until redox determinations, all samples were stored at –80°C.
Determination of redox markers
All the assays were performed in duplicate samples. The absorbance/florescence was measured using Infinite M200 PRO Multimode Microplate Reader (Tecan). The results were standardized to 100 mg of total protein. The content of total protein was estimated colorimetrically at 562 nm wavelength via the bicinchoninic acid (BCA) method. A commercial kit was used according to the manufacturer’s instructions (Thermo Scientific PIERCE BCA Protein Assay; Rockford, IL, USA).
Redox status
To assess the redox status, TAC, TOS, and OSI were evaluated.
The TAC level was determined using the colorimetric method by measuring changes in ABTS•+ (2,2 -azino-bis-3-ethylbenzothiazoline-6-sulfonate) absorbance at 660 nm [20]. The TOS level was analyzed using the colorimetric method by measuring the oxidation of ferrous ion to ferric ion in the presence of oxidants in a sample; OSI was calculated using the formula: OSI = [TOS]/[TAC] × 100%.
Malondialdehyde
The concentration of MDA was determined colorimetrically at 535 nm using the thiobarbituric acid reactive substances (TBARS) method. 1,3,3,3-tetraethoxypropane was used as a standard.
TAC/MDA ratio
The plasma TAC/MDA ratio was calculated as an index of oxidative status by dividing total antioxidant capacity by the malondialdehyde level.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 8.4.3 for Windows (GraphPad Software, La Jolla, USA). The Shapiro-Wilk test was used to examine normal distribution. For normal distribution of the results, the Student’s t-test was used. In non-normal distribution of the results, the Mann-Whitney U test was used. The correlations between the measured parameters were analyzed by Pearson correlation coefficient. Statistical significance was established at p < 0.05.
Results
Adenocarcinoma was found in all 50 cases. According to the TNM classification, the patients were categorized as follows: 1 patient had a T1 lesion, 39 patients were classified as T2, and the remaining 10 patients had T3. Thirty patients did not have lymph node metastasis (N0), in another 10 metastases from 1 to 3 lymph nodes (N1a, N1b) or deposits from neoplastic cells (N1c) were found. The remaining 10 patients had involvement of more than 3 lymph nodes (N2). Distant metastases, i.e. the feature of M1, were found in 8 patients, no distant metastases were found in the remaining 32 patients.
Nutritional status based on BMI
In the study group, according to BMI index, no underweight patients were found, 18 patients had normal body weight, and the remaining 32 patients were overweight or obese (with a significant prevalence of obesity).
Nutritional status assessment based on the NRS 2002 scale
In 12 patients, the sum of points was 3, which indicates a proper nutritional status. However, in the remaining 38 respondents, this result was 4 points or more, which may indicate malnutrition.
Total antioxidant/oxidant status
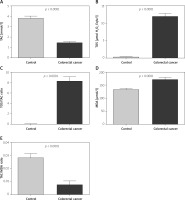
To assess the redox status, we focused on TAC and TOS. Furthermore, we also calculated OSI by dividing the TOS level by the TAC level (TOS/TAC ratio). In the plasma of patients with colorectal cancer, the TAC level was significantly lower compared to the control group (p < 0.0001). We observed a considerable increase in TOS in the plasma of the study group patients compared to healthy controls (p < 0.0001). The OSI value in colorectal cancer patients was significantly higher than in the healthy controls (p < 0.0001) (Figure 1).
Oxidative damage products
To assess the oxidative stress, we used oxidative damage products of lipids (MDA). There was a statistically significant increase in the MDA concentration in plasma of colorectal cancer patients compared to the MDA concentration in the control group (p < 0.0001) (Figure 1).
Comparison of TAC, TOS, OSI depending on the chosen nutrition indicators
We demonstrated a considerably higher TAC level (p = 0.0017) and a considerably lower TOS level in patients with CRC with normal BMI (≤ 24.9 kg/m2) compared to those with increased BMI (> 24.9 kg/m2) (Figures 2 A–E). TAC was significantly higher whereas TOS, OSI and TAC/MDA ratio were significantly lower in patients without symptoms of malnutrition than in patients with malnutrition (p = 0.0344, p = 0.0174, p = 0.0358, p = 0.0200, respectively) (Figures 2 A–E).
Discussion
Oxidative stress, malnutrition and inflammatory response are changes which accompany cancer. Malnutrition and cachexia and their roles in cancer have been questioned by significantly influencing the treatment choice and patient’s survival [6, 8, 10–16]. The influx of studies describing the influence of oxidative stress on the development of neoplasms such as breast cancer, lung cancer, esophageal cancer, thyroid cancer, or a group of neoplasms that are of interest to the authors of the above work, i.e. colorectal cancer, pancreatic cancer, or stomach cancer [8, 10–16].
The breast cancer study by Feng et al. [8] is a reliable study on the effects of oxidative stress regarding the cancer process. The authors transparently proved the differences in the oxidative stress intensity in patients with breast cancer in comparison to a group of patients with benign lesions and a group of healthy volunteers. The study looked at the levels of TAS, TOS, and OSI in the blood serum. TOS and OSI levels were significantly higher in patients with diagnosed cancer compared to the group with benign lesions and to the group of healthy volunteers. The opposite situation was noted in relation to the TAS level, which was significantly lower in cancer patients compared to the other two groups. The situation was similar in the case of esophageal cancer. Huang et al. [16] presented a study comparing TAS, TOS and OSI levels in 92 patients with esophageal cancer vs 64 healthy volunteers. Additionally in this case, statistically significant differences in the levels of TOS, TAS and OSI between both groups were confirmed. Again, it was found that the levels of TOS and OSI are significantly higher and the levels of TAS significantly lower in the group with esophageal cancer compared to healthy volunteers.
Furthermore, the influence of oxidative stress on the carcinogenesis process in colorectal cancer is becoming more certain [6, 12, 14, 15]. The available literature clearly emphasizes the difference in the levels of TOS, TAS, OSI, and MDA in patients with colorectal cancer compared to healthy people [6, 12, 14, 15]. Based on our own research, we can unequivocally confirm the previously described observations, namely, after comparing a group of 50 patients with diagnosed colorectal cancer to a group of 40 healthy volunteers; we also found that there is a statistically significant difference (p < 0.05) in the levels of TAC, TOS, OSI, MDA and TAC/MDA. In healthy patients, the level of TAC and TAC/MDA is much higher compared to the group of cancer patients. The opposite is true if we take into account the levels of TOS, OSI and MDA. In this case, these levels are significantly higher (p < 0.05) in cancer patients compared to the group of healthy patients. This clearly supports the intensification of oxidative stress in the group of patients with colorectal cancer.
Another investigated factor was the assessment of the severity of oxidative stress in patients with colorectal cancer depending on the nutritional status. In this case, patients with colorectal cancer were divided into groups depending on BMI and the number of points obtained according to the NRS 2002 scale. With respect to BMI, we found a significantly higher level of TAC (p < 0.05) in patients with BMI > 24.9 kg/m2 compared to overweight or obese patients, i.e. with BMI < 24.9 kg/m2. There were no patients in the study group who could be classified into the group of patients with malnutrition on the basis of BMI. However, the TOS values were opposite, i.e. they were significantly higher (p < 0.05) in the group of patients with BMI > 24.9 kg/m2 compared to patients with BMI < 24.9 kg/m2. This observation seems to indirectly confirm two facts: overweight and obesity are factors predisposing to colorectal cancer and obesity is one of the factors which intensify oxidative stress.
The study also used the assessment of the nutritional status of patients based on the NRS 2002 scale. In this case, it was found that in patients with features of malnutrition, TOS and OSI values are statistically significantly higher (p < 0.05) than in patients with normal nutritional status. On the other hand, in patients with normal parameters of nutritional status according to the NRS 2002 scale, significantly higher values of TAC and TAC/MDA were observed compared to patients with features of malnutrition. Based on these observations, it can be concluded that in patients with malnutrition in the course of cancer, oxidative stress is increased.
Conclusions
Malnutrition and oxidative stress appear to be the phenomena implicated in colorectal cancer. Neoplastic disease, such as colorectal cancer, precipitates an increase in oxidative stress. Concurrently, the nutritional status of patients, especially malnutrition, further intensities this process.