Introduction
Since ancient times, a variety of plants have been used in pharmaceutical, food, and cosmetic industries, and traditional medicine due to the presence of bioactive compounds. The species vera belonging to the genus Aloe and the family Asphodelaceae is one of the most discussed monocotyledon medicinal and herbal plants. Aloe vera, which is considered the most potent species, is a popular, and widely grown ornamental perennial succulent plant consisting of two components: the latex (produced under the epidermis of the leaf and is yellow in color and bitter in taste) and the gel (a colorless and tasteless substance produced from the parenchymal cells of the leaves). Both latex and gel possess many bioactive compounds such as amino acids, saponins, anthraquinones, enzymes, monosaccharides, polysaccharides, steroids, and vitamins (Laux et al., 2019). Previous studies have proven various medical and commercial uses of A. vera, including in skin treatment and as an antimicrobial, immune-stimulating, anti-inflammatory, and even anticancer agent (Jadhav et al., 2020).
Although A. vera has excellent potential as a medicinal plant due to its pharmacological attributes in local and international markets, its cultivation is still rudimentary owing to propagation difficulties. Sexual reproduction via seeds is almost ineffective due to male sterility. Therefore, the only feasible propagation strategy is vegetative propagation through sucker or offshoots, which cannot meet the ever-increasing demand for Aloe as it is too slow (Abdi et al., 2013). Hence, there is an urgent need for quick, handy, and alternative Aloe mass propagation techniques. Micropropagation through tissue culture could be an alternative and rapid approach for overcoming the existing limitations in Aloe propagation (Dwivedi et al., 2014; Razib et al., 2016; Niguse et al., 2020). In this technique, plant tissues or organs are used to rapidly multiply, genetically improve, obtain disease-free clones, and conserve valuable germplasms (Molsaghi et al., 2014). Generally, the success of micropropagation is influenced by various factors such as explants, culture media, plant growth regulators (PGRs), culture environment, plant genotypes, and environmental conditions which need to be optimized according to the species for which micropropagation is performed (Chen et al., 2019). Successful micropropagation also depends on the careful selection of explants. For micropropagation of Aloe, various kinds of explants such as meristems, shoot tips, apical buds, axillary shoot segments, leaf segments, rhizomatous stems, and suckers are used. Previous studies have shown that shoot tips are the best explants for the micropropagation of Aloe (Zakia et al., 2013; Molsaghi et al., 2014).
In addition, PGRs, particularly auxins and cytokinins, play a fundamental role in plant growth and development in vitro (Nowakowska et al., 2019). The plant naturally synthesizes PGRs; however, for better growth and enhanced synthesis of metabolites, mainly bioactive molecules, it is essential to add external growth hormones (Singh, 2018). The selection of appropriate combinations of PGRs is the most crucial aspect in establishing a successful protocol for tissue culture (Das et al., 2018). However, the optimum concentration and/or combination of PGRs varies from plant to plant and from genotype to genotype (Molsaghi et al., 2014). The individual effects of various PGRs in efficient micropropagation of Aloe were reported in several studies (Lavakumaran and Seran, 2014; Hailu et al., 2020). Interestingly, 6-benzylaminopurine (BAP) alone was proved to be more effective than the combination of BAP and naphthaleneacetic acid (NAA) for in vitro propagation of A. vera (Danial et al., 2019). In contrast, combinations of PGRs (BAP and NAA) were also shown to be effective for optimal in vitro shoot proliferation in A. vera and Aloe adigratana Reynolds (Daneshvar et al., 2013; Niguse et al., 2020). Moreover, the maximum shoot bud induction in A. vera was achieved with a combination of BAP and NAA (Jakhar et al., 2020).
Besides, thidiazuron (TDZ) was recognized as a highly active and less used cytokinin in the stimulation of shoot organogenesis of various plants, including medicinal and endangered plants (Deepa et al., 2018; Zhang et al., 2021). Some studies have demonstrated that TDZ is more effective than other cytokinins (Bhattacharyya et al., 2016; Yücesan, 2018). In a recent study, a low dosage of TDZ more effectively induced multiple shoot buds of A. vera in a short period than BAP (Seran and Ahmad, 2018). On the other hand, a combination of TDZ and NAA was found to be essential for shoot organogenesis in the case of the endemic medicinal plant Gymnostachyum febrifugum Benth. (Silpa and Thomas, 2021). However, TDZ was used as a cytokinin for shoot proliferation in A. vera (Lavakumaran and Seran, 2014; Seran and Ahmad, 2018). Therefore, the present study aimed to optimize the concentrations and combinations of PGRs, including TDZ, in the culture medium for in vitro micropropagation of A. vera.
Materials and methods
Collection and surface sterilization of plant materials
Healthy and disease-free A. vera plants were collected from Bogura District, Bangladesh, and were identified by the last author (Professor Dr. Fahmida Khatun, Department of Biotechnology, Bangladesh Agricultural University, Bangladesh) of this study. The voucher specimen was preserved at the herbarium of Botanical Garden, Bangladesh Agricultural University, Bangladesh.
In this study, shoot tips and immature leaf segments of A. vera were used as explants. Explants were surfacesterilized with Tween 20 for 10 min by vigorous shaking. Then, they were kept in 1% (w/v) solution of autostin for 45 min, followed by a 30 min treatment with ascorbic acid (0.5 mg/ml). After treatment with autostin and ascorbic acid, the explants were treated with 1% sodium hypochlorite for 3–5 min and then rinsed 3–4 times with sterilized distilled water. Finally, the explants were trimmed to 1.5–2.0 cm and inoculated in a 100 ml glass vial (one explant per vial) containing 25 ml of culture medium.
PGRs
Various PGRs, namely BAP (Sisco Research Laboratories Pvt. Ltd., India), TDZ (SRL, India), NAA (SRL, India), and IBA (SRL, India), were used in this study. Different concentrations of BAP (0.5, 1.0, 2.0, and 4.0 mg/l) and TDZ (0.5, 1.0, 2.0, and 4.0 mg/l) along with 0.5 mg/l NAA were supplemented separately into the Murashige and Skoog (MS) medium in order to optimize their effect on the shoot proliferation of A. vera plant (Table 1). Similarly, different concentrations of IBA (1.0, 2.0, and 3.0 mg/l) were used to determine their effect on root formation. The MS medium without PGRs was used as the control.
Table 1
Different concentrations and combinations of PGRs used for shoot proliferation of A. vera
Culture medium and conditions
As the basal medium, the MS medium (Duchefa Biochemic, Netherland) containing 3% (w/v) sucrose (SRL, India), 10 mg/l citric acid, and 8 g/l agar (HiMedia Laboratories Private Limited, India) was used throughout the experiments. Citric acid was used to control the effect of browning in Aloe (Kumar et al., 2016; Meziani et al., 2016). The pH of the medium was adjusted to 5.8 before autoclaving at 121°C and 15 psi for 20 min. All the cultures were incubated in an incubation room and maintained at an 8/16 h photoperiod with a light intensity of 35 μmol ∙ m-2 s-1 at 25±2°C.
Shoot proliferation
As described in Table 1, shoot tips and immature leaf segments were inoculated as explants on the MS medium with different concentrations and combinations of PGRs. Each treatment consisted of 10 replicates with one explant per replication and was conducted in triplicate. The following data were recorded: the days to shoot induction, the percentage of explants showing shoot induction, the number of shoots, and shoot length (cm).
Rooting of microshoots
For root induction, regenerated microshoots were separated from the shoot clump and inoculated on rooting media. The experiment was conducted in triplicate, and each treatment consisted of 10 replications with one explant per vial. The MS medium supplemented with different concentrations of IBA was used for root induction, whereas the medium without PGR was used as the control. The following data were recorded: the days to root induction, the percentage of explants showing root induction, the number of roots, and root length (cm).
Hardening
After proper root formation was achieved, healthy plantlets were taken out from the medium and washed in running tap water to remove the nutrient media, in order to avoid the fungal attack on the root system. Hardening was the continuation of the micropropagation process, which was conducted in triplicate with a total of 70 plantlets in each case. No treatment concentrations and combinations were considered in this step. The plantlets were then planted in pots containing a mixture of soil and compost (2 : 1). The pots were kept in the shade and sprayed with water twice a day. Then, the plantlets were transferred to large pots and gradually adapted to the outdoor conditions, as described by Razib et al. (2016).
Statistical analysis
All sample selection and measurement procedures were performed blinded until analysis. The experiment followed a completely randomized design, and statistical analysis was carried out by one-way analysis of variance followed by Duncan’s multiple-range tests (DMRT) using the Statistical Package for the Social Sciences software, version 20 (Jinn, 2011). All the results were presented as mean ± standard error (SE), and P -values < 0.05 were considered significant.
Results
Shoot proliferation
Different hormonal supplements, namely cytokinins (BAP and TDZ) and auxin (NAA), were added to the MS medium. Leaf segments and shoot tips were used as initial explants (Fig. 3A and Fig. 3B), but the former failed to produce any response. Therefore, the results are presented for in vitro micropropagation using shoot tips alone.
Fig. 1
Effects of various combinations of BAP and TDZ along with NAA on A) the number of shoots and B) the length of shoots per explant (cm) of A. vera (data were taken after 6 weeks of inoculation); in the graph, the mean values with the same letter(s) do not differ significantly, whereas the mean values with different letters differ significantly (as per DMRT) at 5% level of probability
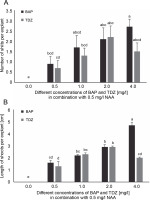
Fig. 2
Effects of various concentrations of IBA on A) the number of roots and B) the length of roots per explant (cm) in A. vera (data were taken after 4 weeks of inoculation); in the graph, the mean values with the same letter(s) do not differ significantly, whereas the mean values with different letters differ significantly (as per DMRT) at 5% level of probability
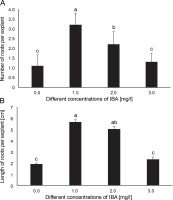
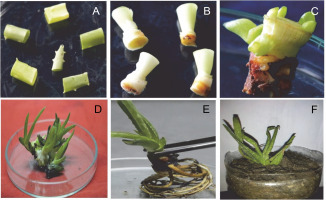
Fig. 3
In vitro micropropagation of A. vera : A) explants – leaf segments, B) explants – shoot tips, C) induction of shoots, D) proliferated shoots, E) rooted plantlet, and F) hardened plant
Days to shoot induction
As presented in Table 2, the number of days to shoot induction was influenced by different concentrations of BAP and TDZ, in combination with a constant concentration of NAA. New buds started to appear from the explants within 5 ± 0.33 to 25 ± 0.49 days of inoculation. Explants inoculated on the BAP-supplemented MS medium needed less time for shoot induction compared to those inoculated on the TDZ-supplemented medium (Table 2). The MS medium supplemented with 4.0 mg/l BAP + 0.5 mg/l NAA showed the best result for shoot induction within minimum number of days (5 ± 0.33) of inoculation (Fig. 3C). Moreover, shoot induction in the medium supplemented with 2.0 mg/l BAP/TDZ + 0.5 mg/l NAA occurred in a statistically identical number of days (7 ± 0.26 and 8 ± 1.42, respectively). The most extended period (24 ± 0.73 and 25 ± 0.49 days, respectively) of shoot induction was observed in the medium in which low amounts of BAP and TDZ (0.5 mg/l each) were combined with 0.5 mg/l NAA. Importantly, explants did not show any sign of shoot proliferation in the control medium lacking the PGRs (Table 2).
Table 2
Effects of PGRs on shoot proliferation of A. vera after 6 weeks of culture
Percentage of explants responding to shoot induction
The best response for shoot induction (90 ± 1.29%) was achieved when 4.0 mg/l BAP + 0.5 mg/l NAA was added to the basal medium (Table 2). The second best response (70 ± 1.05% and 70 ± 1.29%) was observed in the explants that grew in the MS medium supplemented with the same concentration of BAP and TDZ (2.0 mg/l) combined with 0.5 mg/l NAA. However, these explants responded statistically similar at 1.0 mg/l and 4.0 mg/l of TDZ (60 ± 1.56 and 60 ± 1.48%). The lowest response (30 ± 0.93%) was observed in the explants that grew in the MS media supplemented with 0.5 mg/l TDZ + 0.5 mg/l NAA. In general, regeneration of shoot buds was lower in the medium supplemented with TDZ compared with the medium supplemented with BAP. The shoot tips that were cultured on the medium without phytohormone failed to produce any new shoots (Table 2).
Number of shoots produced on media with different combinations of PGRs
A significant difference in the number of shoots produced by the explants was found in the media containing different amounts of BAP and TDZ, combined with NAA. It was found that the explants cultured on the MS medium supplemented with 4.0 mg/l BAP + 0.5 mg/l NAA yielded the significantly highest number of shoots (2.7 ± 0.36) than any other treatment (Fig. 1A and Fig. 3D). No significant differences were observed in the number of the shoots in the case of explants cultured in the medium containing 2.0 mg/l BAP/TDZ with 0.5 mg/l NAA (2.1 ± 0.54 and 2.2 ± 0.53 shoots per explant, respectively). The least number of shoots per explant (0.7 ± 0.36) was observed in the MS medium augmented with 0.5 mg/l TDZ + 0.5 mg/l NAA (Fig. 1A), and no shoots were formed in the control medium.
Shoot length
The significantly highest shoot length (4.7 ± 0.25 cm) was found in the explants cultured in the MS medium containing 4.0 mg/l BAP + 0.5 mg/l NAA (Fig. 1B). However, the high concentration of TDZ (4.0 mg/l TDZ + 0.5 mg/l NAA) retarded the growth of shoots and resulted in their malformation. No statistical differences were found in shoot length between the explants growing in the MS medium supplemented with 1.0 mg/l BAP/TDZ + 0.5 mg/l NAA and 2.0 mg/l BAP/TDZ + 0.5 mg/l NAA (Fig. 1B).
Root induction
To observe root induction, individual regenerated microshoots were inoculated on the MS medium supplemented with different concentrations of IBA (1.0, 2.0, and 3.0 mg/l).
Number of days to root induction
Root induction of the regenerated microshoots occurred within 3 weeks of inoculation on the rooting medium. The microshoots that grew on the MS medium supplemented with 1.0 mg/l IBA required the minimum time (11 ± 0.79 days) to emerge the root. On the other hand, the microshoots that grew on the MS medium supplemented with 2.0 mg/l IBA and 3.0 mg/l IBA required more time (17 ± 1.06 and 15 ± 1.67 days, respectively) for root induction. Similarly, the medium without growth regulators required the maximum number of days (21 ± 1.21) for root induction (Table 3).
Percentage of explants responding to root induction
Responses of the explants to root induction ranged from 30 ± 0.95 to 80 ± 1.97%. The highest rooting response (80 ± 1.97%) was observed with the medium enriched with 1.0 mg/l IBA. Thereafter, the percentage of explants that responded to root induction decreased with the increasing concentration of IBA, and the lowest percentage of root induction (30 ± 0.95%) was observed in the basal MS medium without PGRs (Table 3).
Number of roots
A statistically significant difference was observed in the number of roots produced per explant due to the effect of IBA concentrations. The highest number of roots (3.2 ± 0.57) per explant was observed in the explants that grew on the medium supplemented with 1.0 mg/l IBA (Fig. 2A). Thereafter, the number of roots decreased with the increasing concentration of IBA. The lowest number of roots (1.1 ± 0.57) was observed in the hormone-free (control) medium.
Root length
Root length was significantly influenced by the concentration of IBA. The medium supplemented with 1.0 mg/l IBA induced the highest root length (5.67 ± 0.21 cm) (Fig. 2B and Fig. 3E). Root length reduced with increasing concentration of IBA. In contrast, the MS medium without phytohormones showed the lowest root length (1.92 ± 0.09 cm) (Fig. 2B).
Hardening
The plantlets with actively growing roots were selected for hardening. Initially, 210 in vitro -grown plantlets (70 plantlets from each case) were transplanted for hardening, and among these, 187 plantlets (62, 58, and 67, respectively) were hardened (Fig. 3F). The survival rate of the plants was recorded at 82% (Table 4).
Discussion
In vitro micropropagation is a viable method for the mass production of healthy plants. Successful micropropagation depends on various factors, such as the plant genotype, culture medium, PGRs, culture environment, and explant developmental stage (Chen et al., 2019). In the present study, shoot tips and leaf segments were initially used as the explants for in vitro micropropagation. Unfortunately, leaf segments did not survive due to browning or blacking (visual observation), which are generally related to the release of phenolic chemicals from the cut surfaces of the explants (Nayanakantha et al., 2011). Therefore, the present study focused on shoot tips alone. As proposed in previous reports, shoot tips are the most efficient explants for in vitro micropropagation of Aloe (Lavakumaran and Seran, 2014; Razib et al., 2016). This might be attributed to the fact that juvenile tissues such as shoot tips have a remarkable regeneration ability (Lardon and Geelen, 2020).
Generally, shoot proliferation is influenced by numerous PGRs. Bernula et al. (2020) have reported the necessity of using both cytokinins and auxins (a high cytokinin-to-auxin ratio) for shoot proliferation in Arabidopsis. In the present study, shoot proliferation was enhanced by increasing the concentration of BAP along with 0.5 mg/l NAA. The best shoot proliferation was achieved from the explants inoculated on the MS medium containing 4.0 mg/l BAP + 0.5 mg/l NAA (Fig. 1). This might be because the synergism of these concentrations works well with the endogenous and exogenous levels of cytokinins and auxins, genotype, age, and the explants used (Das and Bora, 2018). In the case of TDZ combinations, shoot proliferation was also enhanced on the medium supplemented with TDZ at concentrations up to 2.0 mg/l (Fig. 1). However, a further increase in the TDZ concentration (4.0 mg/l), together with the supplementation of the medium with NAA, resulted in the production of shoots with developmental defects. Overall, the trends of microshoot production in the shoot tip of the explants are consistent with the findings of Lavakumaran and Seran (2014), who tested different concentrations and combinations of BAP and TDZ along with NAA in in vitro shoot organogenesis of A. vera using shoot tips. The highest number of microshoots from A. vera shoot tips was achieved on the medium supplemented with 3.0 mg/l BAP + 0.5 mg/l NAA. Similarly, the explants cultured on the medium containing 1.0 mg/l TDZ + 0.5 mg/l NAA also produced microshoots, whereas those cultured with 3.0 mg/l TDZ + 0.5 mg/l NAA showed severe abnormalities. Niguse et al. (2020) observed that the combinations of BAP or TDZ and NAA were effective in inducing shoot proliferation and multiplication in Aloe. In the present study, the highest shoot proliferation was observed with the medium supplemented with 4.0 mg/l BAP + 0.5 mg/l NAA.
However, the auxins NAA, IAA, and IBA effectively induced roots on microshoots of A. vera L. (Zakia et al., 2013; Dwivedi et al., 2014). IBA is considered more stable and effective in inducing adventitious roots in clonal propagation (Wei et al., 2019). Higher concentrations of IBA were reported to enhance the rooting of A. adigratana Reynolds when the half-strength MS medium supplemented with different concentrations of IBA and NAA was used as the rooting medium (Niguse et al., 2020). In the present study, the hormone-free medium (control) and media supplemented with different concentrations of IBA (1.0, 2.0, and 3.0 mg/l) were used for root induction. The explants cultured in the medium containing a low IBA concentration (1.0 mg/l) showed a better response for inducing roots than those cultured in the hormone-free medium (Fig. 2). This finding is consistent with the earlier report of Daneshvar et al. (2013), in which the best root induction was observed at the concentration of 1.0 mg/l of IBA than in the auxin-free medium. Nevertheless, root induction was found to gradually decrease when the exogenous IBA concentration (more than 1.0 mg/l) in the culture medium was increased further. Interestingly, the hormone-free medium produced roots on microshoots perhaps due to the presence of endogenous auxins (Amiri and Mohammadi, 2021). In addition, Thaniarasu et al. (2015) studied the shoots of a medicinal plant, Plectranthus bourneae Gamble, rooted in media augmented with different auxins (IAA, IBA, and NAA) and reported that IBA was more effective than others. The stimulatory effect of IBA has also been observed in root induction of Pyrus elaeagrifolia Pallas (Aygun and Dumanoglu, 2015) and Cryptolepis sanguinolenta (Monney et al., 2016). The results of the present study suggest that 1.0 mg/l IBA is the best for in vitro rooting of A. vera.
Furthermore, hardening of the in vitro-grown plantlets is the crucial step before transplanting them to the soil (Shekhawat et al., 2015). Various researchers have reported the successful hardening of A. vera (Kiran et al., 2017; Shibru et al., 2018). In the present study, rooted plantlets were transferred to pots with a mixture of soil and compost (2 : 1). After 1 month, the percentage of survivability was recorded at 82%, which is consistent with other studies that reported 74–85% survival of A. vera (Das and Srivastav, 2015; Jakhar et al., 2020).
Conclusions
The present study revealed that the shoot tip explants of A. vera responded differently to the PGRs added to the culture medium. The combination of 4.0 mg/l BAP (cytokinin) + 0.5 mg/l NAA (auxin) proved to be effective, with the significantly highest number and length of shoots and the highest percentage of explants responding to shoot induction within the shortest time. IBA concentrations significantly influenced the root formation. High-frequency rooting within the minimum number of days, the highest root number, and the highest length were achieved with the medium containing 1.0 mg/l IBA. Thus, the proposed protocol is standardized, reproducible, and straightforward for in vitro micropropagation of A. vera.










