Introduction
The high rate of consumption of different types of poultry species worldwide has increased the demand for a healthier source of food. In particular, the increased interest in achieving a high source of protein, combined with the need to enhance the natural flavor, has made guinea fowl meat a better choice for many, especially for people in sub-Saharan Africa. Guinea fowl (menigridis) possess a drier leaner meat that contains marginally higher amount of protein than chicken and Turkey and slightly lower calories per gram (Abdul-Rahman et al., 2019). In addition to being the meat of interest, guinea fowl has the ability to produce loud alarm calls in case of danger and consume parasites such as ticks in addition to hunting snakes; due to these factors, the local and commercial production of guinea fowl has significantly increased (Vignal et al., 2019). Consequently, the generation of guinea fowl feathers (GFFs) from slaughterhouses, markets, and poultry-processing plants has also increased. The feather constitutes approximately 6% of the total weight of guinea fowl (Mittal, 2006). It is long and black or brown in color, with a distinct white-spotted circular color pattern (Musundire et al., 2017; Ebegbulem, 2018). The colored component of the feather phenotype contains an organic pigment (melanin) derived from a tyrosine residue that is highly cross-linked and resistant to degradation (Tesfaye et al., 2017). GFFs are highly mechanically stable, are recalcitrant to enzymatic and microbial degradation, and often end up in open lands, drainages, and landfills, thus compounding environmental pollution. According to earlier studies, the polluted environment serves as a suitable habitat for infectious bacteria and flies, which can potentially transmit several diseases such as chlorosis, mycoplasmosis, and fowl cholera (Musundire et al., 2017; Foster-Nyarko et al., 2020).
Furthermore, industries persistently release heavy metal pollutants into aqueous and terrestrial environments at a frightening rate, which has been a great concern over the years (Rahimnejad et al., 2012; Parkash, 2016). Identifying a cheap and eco-friendly approach to removing heavy metals from effluents before they pollute aqueous environments has become the biggest challenge for governments and scientists, in order to meet the ever-increasing demand for safer water (Nair et al., 2013). Many researchers have studied the use of chicken feather waste (especially white feather) to remove heavy metals from different types of aqueous environments (Sun et al., 2009; Wang et al., 2016; NoerHidayat et al., 2019). Studies have also adopted different pretreatment methods to enhance heavy metal removal using feathers and other biological wastes, such as chemical, enzymatic, physical, and biological methods, which improve feather digestibility and bioadsorption (Sun et al., 2009; Ndeddy Aka and Babalola, 2016; Tesfaye et al., 2018; Igiri et al., 2018; Fernández-González et al., 2019). However, the use of GFFs as bioadsorbents, as well as their pretreatment to increase their removal properties and hence to enhance their heavy metal selectivity, has not been documented yet.
The heavy-metal-laden GFFs thus generated can take a longer time to degrade naturally, thus necessitating the search for feather-degrading microbes (FDMs) that are capable of degrading heavy-metal-saturated GFFs. The amount of keratinase required for the degradation of heavy-metal-free and heavy-metal-saturated GFFs could vary from one species to another and is influenced by the physical and chemical properties of the species as widely reported (Tork et al., 2010; Sivakumar et al., 2012; Fang et al., 2013; Yusuf et al., 2015, 2016). However, even under optimum conditions, the growth of FDMs may be inhibited if the concentration of heavy metals in the feathers is high. The efficiency of heavymetal-laden GFF degradation could be enhanced through the process of cell immobilization as immobilized cells are more stable than free cells.
This study was the first to use raw and pretreated GFFs in the bioremediation of heavy metals (Cu and Zn). Furthermore, the isolation and selection of a novel bacterium that shows a high potential for degrading Cu- and Zn-laden GFFs in its free and immobilized forms was also attempted. Information on the bioconversion potential of the bacterium under the influence of various physical and chemical factors is provided in this paper.
Materials and methods
Sample collection and processing
GFF wastes were collected from a chicken abattoir in the Nigerian state of Kano. The collected GFFs were spread on clean polythene bags, and non-feather parts such as peak, nails, and excreta were handpicked and removed. GFFs were thoroughly washed with tap water and detergent, and then their fat remnants were removed by soaking in a solution containing 200 ml of deionized water, methanol, and chloroform in the ratio of 1 : 1 : 10. The cleaned GFFs were further rinsed in distilled water and oven-dried at 80°C for 6 h until a constant weight was achieved (Yusuf et al., 2016, 2019).
Chemical and physical pretreatment of GFFs for adsorption of Cu and Zn
Both physical (steam/autoclaving and boiling) and chemical (NaOH, tannic acid, and acetone) methods were used in the pretreatment of GFFs. Steam (thermal) pretreatment was carried out by autoclaving 5 g of the whole GFFs suspended in 100 ml of distilled water at 121°C and 21 psi for 30 min. GFFs preheated with boiled water for 30 min were also used. In chemical pretreatments, three chemicals were used, viz NaOH, acetone, and tannic acid. NaOH pretreatment of GFFs was carried out according to the method of Sun et al. (2009) using 0.01 mol/l NaOH. In brief, GFFs (10 g) were suspended in 100 ml solution of NaOH and stirred at room temperature for 8 h (Sun et al., 2009). For pretreatment with tannic acid, Ratnakumari and Kota’s (2012) method was used, where 10 g GFFs was suspended in 5% tannic acid solution for 1 h. GFFs in both pretreatments were then separated, precipitated (only in the case of tannic acid), and washed several times with distilled water. In the case of acetone pretreatment, 10 g GFFs was added to 100 ml of 0.6% acetone and incubated at room temperature for 90 min (Mrajji et al., 2019).
Preparation of Zn and Cu stock solutions
As reported in an earlier study, 1000 parts per million (ppm) solutions of Cu and Zn were prepared by dissolving 3.392 g and 4.42 g of CuSO4 and ZnSO4, respectively, in 1000 ml of distilled water (Na deem et al., 2016). From these stock solutions, working solutions were prepared by pipetting an appropriate volume of the stock solution into a volume of distilled water.
Adsorption of Cu and Zn by pretreated and untreated GFFs
This experiment was performed in a batch mode in distilled water (dH2O). Briefly, 100 ml of Cu and Zn working solutions of 15, 25, and 50 ppm final concentrations was taken in appropriately labeled 250 ml Erlenmeyer flasks. In separate flasks containing different concentrations of Cu and Zn, 5 g of each of the pretreated and untreated GFFs was placed. Untreated GFFs kept in Cu and Zn solutions and those in flasks without heavy metals served as control. The flask setup was then kept on a rotary shaker at 150 rpm for 6 h (Tan et al., 2003; Guo et al., 2016). GFFs were separated from metal solutions using No. 1 Whatman filter paper. Using an Atomic Absorption Spectrometer (AAS) (CG-AA-7000 equipment, Shimadzu, Japan), the residual concentrations of Cu and Zn in each flask were determined (Carvalho et al., 2003). To determine the removal percentage of Cu and Zn by GFFs, the difference between the initial and residual Cu and Zn concentrations was used, as shown in Equation 1.
where Co is the initial metal ion concentration (mg/l), Ct is the metal ion concentration at time t (mg/l), and %R is the percentage removal.
Optimization of physical conditions affecting Cu and Zn adsorption by GFFs
Using a one factor at a time (OFAT) approach, the effects of pH, temperature, and amount of GFFs on Cu and Zn adsorption by GFFs were determined. Various amounts of GFFs measured in g/l (1, 2, 3, 4, and 5) were placed in separate flasks containing 100 ml of 25 ppm of each Cu and Zn solution, and the incubation temperature and the initial pH of aqueous solutions were maintained at 30°C and 7.5, respectively. The effects of pH on Cu and Zn adsorption by GFFs were determined by adjusting the initial pH of the aqueous solutions to 2, 4, 6, 8, and 10 while keeping GFF concentrations at the optimum amount determined earlier and temperature at 30°C. The incubation temperature was then varied (25, 30, 35, 40, 45, 50, 55, and 60°C) while maintaining other parameters at the optimum levels. The residual Cu and Zn in all flasks were determined using an AAS, and the optimum conditions were used in the subsequent experiments.
Isolation of GFF-degrading bacteria (GFF-DB) and preparation of culture media
Seven GFF-DB were obtained from two sources: 1) three from previous collections of feather-degrading bacteria (FDB) in the laboratory and 2) four from soil samples collected from three guinea fowl slaughter and farm locations. These samples were collected and processed as previously described (Yusuf et al., 2016, 2019). In brief, 1 g of the soil samples was suspended in 0.9% saline solution, vortexed for 2–3 min intermittently, and centrifuged at 1000 rpm for 5 min. The supernatant from each tube was inoculated in a 250 ml Erlenmeyer flask containing 100 ml feather meal broth (FMB) and supplemented with GFFs as a sole source of carbon and nitrogen. FMB was prepared as described earlier by Yusuf et al. (2016, 2019, 2020) by dissolving 0.5 g/l of NaCl, 0.7 g/l of K2HPO4, 1.4 g/l of KH2PO4, and 0.001 g/l of MgSO4 ∙ 6H2O in 1000 ml of distilled water, and the pH of the mixture was adjusted to 7.5. The inoculated FMB were incubated on an orbital shaker operating at 150 rpm at 30°C. Bacteria from flasks that showed high degradation of GFFs were isolated through inoculation of 1 ml of FMB on feather meal agar (FMA), pH 7.5, which contained 1.0 g/l feather, 0.5 g/l NaCl, 0.7 g/l K2HPO4, 1.4 g/l KH2PO4, 0.001 g/l MgSO4 ∙ 6H2O, and 1.5 g/l agar. Isolates from the previous collections were inoculated into FMB containing GFFs, and their ability to degrade GFFs was determined as previously described (Yusuf et al., 2020).
Screening GFF-DB for Cu and Zn tolerance
One part per million solutions of Cu and Zn were prepared from their respective stock solutions in FMB, and the bacterial growth in two sets of FMB (FMB with metal and without metal) was compared. One percent suspensions of the collected and the isolated GFF-DB were inoculated into 100 ml FMB containing 5 g/l of GFFs and incubated at 30°C. Any bacteria that grew, degraded feathers faster, and showed a higher keratinolytic activity in the presence of both Cu and Zn were selected and inoculated onto fresh FMA.
Morphological, biochemical, and molecular identification of GFF-DB
Gram staining and Bergey’s manual ofsystematic bacteriology were used to identify the morphological and biochemical characteristics of the bacterium, respectively (Cappuccino and Sherman, 1996; Brenner et al., 2005). Molecular identification was carried out using 16S rRNA sequence analysis, as previously described (Yusuf et al., 2020).
Biodegradation of Cu- and Zn-polluted GFFs
Biodegradation of Cu- and Zn-laden GFFs by free cells of strain IY-BUK1 was carried out in FMB containing 5 g/l of Cu- and Zn-laden GFFs as substrates. Cu- and Zn-free GFFs placed in another FMB and flasks that contained Cu- and Zn-laden GFFs but without GFF-DB served as control. The flasks were incubated on a rotatory shaker at 150 rpm for 7 days at 30°C. At the end of the incubation period, the percentage of feather degradation was calculated, as previously described (Yusuf et al., 2016). The growth of the GFF-DB in each flask was estimated in duplicate in colony-forming units (CFU).
Optimization of factors influencing keratinase production and degradation of Cu- and Zn-laden-GFFs by selected GFF-DB
To determine how pH, temperature, inoculum size, and extra carbon and nitrogen influence keratinase production and degradation of Cu- and Zn-laden GFFs, OFAT and response surface methodology (RSM) were employed. Effects of pH (2, 4, 6, 8, and 10), temperature (25, 30, 35, 40, 45, and 50°C), inoculum size (1, 2, 3, 4, and 5%), extra carbon source (glucose, sucrose, and maltose), and extra nitrogen source (ammonium chloride (AMC), ammonium sulfate (AMS), urea, skim milk, and yeast extract) were optimized using OFAT, as described earlier (Yusuf et al., 2016).
For RSM, Plackett–Burman factorial design (PBFD) was used to select the major factors required for the maximum keratinase yield. The effects of GFF concentration, pH, temperature, and inoculum size on keratinase production were investigated at two levels (minimum and maximum). Design Expert software, version 6.0 (Stat Ease, Minneapolis, USA), was used to generate 12 sets of experiments that were conducted in triplicate. Effects of each factor on keratinase production were determined by the analysis of variance (ANOVA) using the calculated p-value of each factor.
To design and check the interactions between the factors selected with PBFD, central composite design and RSM were used. Three factors coded as A, B, and C were studied at four levels in triplicate. The factors included temperature (35–40°C), inoculum size (2–4%), and GFF concentration (2–4 g/l). Twenty experimental runs were included in the design. The response (y ), which is the average keratinase production for each run, was fitted to a second-order polynomial equation, and a model equation that relates to the independent factors (A–C) was generated as shown in Equation 2.
where y is the predicted response of keratinase production), βo is the intercept, β i is the linear coefficient, β ij is the quadratic coefficient, and X represents the factors tested (in this case, A, B, and C). The P -values and confidence levels of the data obtained were calculated using the ANOVA tool embedded in Design Expert software, version 6.0.6. The quality of the model was ascertained statistically by the coefficient of determination R 2, and its statistical significance was determined using F -test values. Using 3D plots, optimal values from the linear and interactions of factors were estimated. The predicted values from RSM were validated by experiments conducted in triplicate.
Immobilization of GFF-DB in gellan gum
According to the method employed in a previous study, immobilization of the selected GFF-DB bacterium in the gellan gum matrix was carried out using melanized black feathers (Yusuf et al., 2020). By dissolving 0.75 g of gellan gum powder in 100 ml of distilled water, a 0.75% (w/v) suspension of gellan gum powder (Sigma) was prepared, which was then heated to 75°C on a hot plate for 5 min. Then 0.06 g of calcium chloride was added to the gum suspension, and the mixture was stirred continuously until the suspension cooled down to 45°C. By adding drops of 0.1 M NaOH solution, the pH of the gellan gum + CaCl2 solution was adjusted to 7, and then, 3.5 g of GFF-DB pellet was added, and the mixture was continuously stirred. Using a peristaltic pump connected to a narrow tubule, beads with an average size of 2–3 mm were produced and allowed to drop in 500 ml of canola oil containing 0.1% Span 80 (Sigma). The beads were separated from the canola oil using a 0.5-mm mesh, kept in distilled water, and refrigerated for 2 h. The refrigerated beads were then washed several times with 0.1% (v/v) Tween 80 solution and distilled water and stored at 4°C until future use.
Determination of keratinase activities and bacterial growth
Every 24 h, an aliquot of 10 ml hydrolysate was collected from FMB and centrifuged at 10 000 rpm for 15 min. For measuring keratinase activities, the cell-free supernatant was used and soluble keratin was used as a substrate as previously described (Wawrzkiewicz, 1987). Bacterial growth was estimated using the platecounting method in CFU/ml (Yusuf et al., 2016).
Statistical analysis
A completely randomized design was used in this study. All experiments were run in triplicate, except where indicated. To check for errors and variations, means and standard deviations (SD) were determined from the repeated experiments, and the values were represented as mean ± SD. Under different conditions, differences in the rate of degradation and keratinase production between the groups were analyzed by Student’s t-test and one-way ANOVA at 95% confidence level using the Minitab (version 16) software package (State College, Pennsylvania). To check the differences in keratinase production and feather degradation under the influence of different heavy metals, Tukey’s post hoc analysis after ANOVA was used.
Results and discussion
Chicken feathers as an adsorbent for different wastes including heavy metals have been used in previous studies, and their sorption capacities for metal ions have been found to vary with different pretreatment methods. However, no work has studied the removal capacity of GFFs and the effects of different pretreatments on their ability to enhance digestibility before bioadsorption of Cu and Zn metals. The results of the analysis of effects of each pretreatment method on GFFs ability to remove Cu and Zn from aqueous solution are summarized in Table 1. An overall decrease in the GFFs’ ability to remove Cu and Zn was observed as the heavy metal concentration increased from 15 to 50 ppm. The highest Cu and Zn removal was observed with the acetone pretreatment method, followed by the autoclave treatment method against the raw GFFs. NaOH-pretreated GFFs resulted in higher removal of Cu at 15 ppm (98%), 25 ppm (83%), and 50 ppm (88%) than Zn removal. In support of this finding, thermal alkaline pretreatment has recently been reported as a promising method for enhancing the digestibility of chicken feathers in the production of a protein-rich hydrolysate (Cheong et al., 2018). Destruction of the hard proteins in GFFs by heating in the autoclave process could have facilitated their porosity, leading to higher adsorption of the heavy metals (Wang et al., 2018).
Table 1
Comparison of the effects of different pretreatment methods of GFFs on removal of different concentrations of Cu and Zn
Based on this observation, acetone pretreatment was chosen for subsequent experiments involving Cu and Zn. Factors that could influence Cu and Zn uptake by acetone-treated GFFs, including pH of the aqueous solution, temperature, and GFF concentration, were analyzed. The metal removal capacity of acetone-treated GFFs was influenced by a change in the pH of the medium. As shown in Figure 1A, at pH 4 (81.3%) and 6 (84.3%), the percentage removal of Cu and Zn was optimum, respectively. Although slight adsorption of Cu and Zn by GFFs occurred at an initial pH of 8, no significant adsorption (24%) was observed at pH 2 for Zn.
Fig. 1
Optimization of pH (A), temperature (B) and GFF concentration (C) on adsorption of Cu and Zn in aqueous solution by acetone pretreated GFF
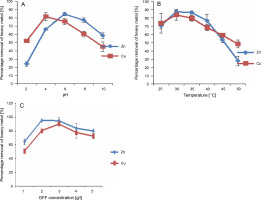
The low removal capacity at pH 2 and 3 may be attributed to the high concentration of H+ ions in the solution. Optimal removal of Cu and Zn occurred at a temperature of 30°C (87.5% for Zn and 83.5% for Cu), which gradually decreased at 40°C (76% for Zn and 68% for Cu) (Fig. 1B).
To identify the substrate concentration at which the highest removal of Zn and Cu occurred, the concentrations of GFFs were screened. Percentage removal of Cu and Zn reached approximately 90% with 2–3 g/l of GFFs and slightly decreased as GFF concentration reached 4 g/l (83.5% for Zn and 77% for Cu) and beyond (Fig. 1C).
At optimum pH, temperature, and GFF concentration, acetone-treated GFFs polluted with Cu and Zn were generated, which were used as a substrate for keratinase production by GFF-DB. In this study, three heavy-metaltolerant FDB from previous collections were used (Yusuf et al., 2020). In addition, four GFF-DB capable of utilizing GFFs polluted with either Cu or Zn as a substrate for producing keratinase were isolated from soil samples obtained from GFF dumpsites. Out of the seven bacteria, two bacteria (B and G) effectively degraded GFFs polluted with both Cu and Zn and produced a higher amount of keratinase (Table 2). On the contrary, bacteria B (which was previously used to degrade melanized feathers) grew faster in FMB. It also produced the highest amount of keratinase and degraded GFFs polluted with both Cu and Zn and was therefore selected for further study. This indicated that the bacterium showed a higher preference for melanin-containing feathers as it degraded both melanized chicken feathers and GFFs faster. With an initial pH of 7.0, incubation temperature of 30°C, and 1 g/l of GFFs polluted with Cu and Zn, the selected bacterium produced 38.5 U/ml keratinase and degraded 1 g/l of the polluted GFFs completely in 5 days, which is high when compared with other keratinase-producing bacteria such as Serratia sp. (21 U/ml) and Arthrobacter sp. (13 U/ml) (Barman et al., 2017; Narayanapp et al., 2019).
Table 2
Screening for GFF-degrading bacteria
The bacterium completely degraded 5 g/l of Cu- and Zn-laden GFFs in 5 days, but the degradation began earlier in the flask containing Cu-laden GFFs. However, the biodegradation of Cu- and Zn-laden GFFs was completed in 6 days (result not shown).
The morphological, biochemical, and 16S rRNA sequence analyses of the selected bacterium (Pseudochrobactrum sp. IY-BUK1) have been reported in a previous study (Yusuf et al., 2020). The bacterium, which is Gram-negative, oxidase-positive, non-spore-forming, and nonmotile bacilli, was the first GFF-DB to be reported. Even though Gram-positive bacilli have been widely reported to degrade chicken feathers (Goldstein et al., 2004; Patinvoh et al., 2016; Bhari et al., 2018), Gram-negative bacteria have recently been indicated in the degradation of melanized feathers as well as heavy-metal-polluted feathers (Yusuf et al., 2019).
Optimization of parameters affecting the ability of the bacteria to degrade GFFs and produce keratinase
The ability of Pseudochrobactrum sp. IY-BUK1 to produce keratinase required for degrading GFFs laden with a mixture of Cu and Zn was influenced by the initial pH of FMB, temperature of incubation, the amount of polluted GFFs, bacterial inoculum size, and extra carbon and nitrogen. When the pH of FMB increased from 7.0 to 8.0, keratinase production increased from 80 to 119 U/ml (Fig. 2A). Complete degradation of 1 g/l of Cu- and Zn-laden GFFs was achieved in 4 days at pH 8 as against 5 days when the pH was 7 (result not shown). A gradual decrease in keratinase activity and GFF degradation was observed above pH 8. The decrease in keratinase production and GFF degradation was observed at pH below 8 and above 9, which indicated that the bacteria were slightly inhibited under higher acidic and alkaline conditions. Similar observation was reported for Alcaligenes faecalis AQ005-01 and Bacillus sp., which degraded white unpolluted feathers and melanized heavy-metal-polluted chicken feathers, respectively, at pH 8 (Yusuf et al., 2016, 2020). There are many reports on FDB species indicating that slight acidic to alkaline pH enhanced keratinase activity and feather degradation (Jeong et al., 2010; Pandian et al., 2012; Bhari et al., 2018; Łaba et al., 2018). Similarly, the strain’s capacity to degrade Cu- and Zn-laden GFFs and produce high keratinase was optimal at 40°C (Fig. 2B). Even though Pseudochrobactrum sp. IY-BUK1 was previously reported to selectively degrade black chicken feathers optimally at temperatures slightly above 40°C (Yusuf et al., 2020), FDB have been reported to degrade non-melanized chicken feathers optimally at room temperature (Sangali and Brandelli, 2000; Lo et al., 2012; Tamreihao et al., 2019; NoerHidayat et al., 2019).
Fig. 2
Optimization of pH, temperature, inoculums size, amount of polluted GFF, extra carbon and nitrogen sources for enhanced production of keratinase by Pseudochrobactrum sp. IYBUK-1
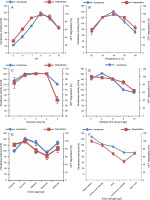
Optimum keratinase activities were achieved within the 3–4% inoculum size (Fig. 2C), which is in agreement with the report of Cai et al. (2008) and contradicts other findings in which 2% inoculum size was the optimum (El-Refai et al., 2005; Cai et al., 2008; Sahoo et al., 2012; Yusuf et al., 2016). The amount of keratinase produced by GFF-DB was also influenced by the amount of heavy-metal-polluted GFFs placed in FMB (Fig. 2D). Even though degradation of Cu- and Zn-polluted GFFs decreased with an increase in the GFF concentration from 2 g/l, keratinase activity was optimal at 4 g/l and decreased further. A lower keratinase production and feather degradation at GFF concentrations above 4 g/l might be attributable to the increase in the Cu and Zn concentration in the FMB, which inhibited bacterial growth and/or keratinase production by GFF-DB. Previous studies have reported feather concentration as an influential parameter for keratinase production by many FDB (Bach et al., 2012; Patinvoh et al., 2016); however, the toxicity of the accumulated heavy metals adsorbed onto GFFs in the present study might have resulted in reduced keratinase production and feather degradation. The effects of 5 g/l extra elemental sources of carbon and 1 g/l extra nitrogen sources on keratinase production and GFF degradation by strain IY-BUK1 were also determined. Findings of this study indicated that the extra amount of different carbon and nitrogen sources in FMB showed varying effects on keratinase yield depending on the types of FDB and substrates used (Cao et al., 2009; Lo et al., 2012). The addition of extra sucrose, glucose, maltose, and mannose suppressed the keratinase activity and degradation of heavy-metal-laden GFFs. This contradicts the previous report in which the strain’s keratinase yield was stimulated by sucrose in the degradation of melanized chicken feathers (Yusuf et al., 2020). This suggests that the strain’s nutritional requirements vary from one substrate to another. Except for the yeast extract, which did not influence the keratinase activity (Fig. 2F), the addition of extra nitrogen sources of urea, AMC, AMS, and skim milk to FMB containing heavy-metal-laden GFFs suppressed keratinase yield and feather degradation. Findings from previous studies have suggested supplementation of FMB with yeast extracts for enhanced FDB growth and keratinase yield (Kainoor and Naik, 2010; Villa et al., 2013).
The parameters that enhanced the strain’s ability to yield higher keratinase and degrade polluted GFFs faster were subjected to Plackett–Burman design to obtain the significant factors among them. Keratinase yields (response) from 12 experimental runs are shown in Table 3A. The ANOVA and p-value for each parameter are presented in Table 3B. Parameters with a P-value of < 0.05 were considered to have a significant effect on the response and were selected for RSM studies. Two of the significant factors—pH and GFF concentration—exerted a positive effect on keratinase production, whereas inoculum size exerted a negative effect. Using RSM, the effects of the interaction between individual significant parameters on keratinase activities with IY-BUK1 were evaluated in 20 experimental runs.
Table 3A
Plackett-Burman experimental design matrix with keratinase production levels
Table 3B
Plackett-Burman experimental design matrix with keratinase production levels
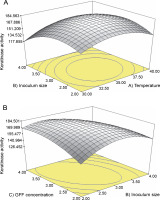
As shown in Table 4, the average keratinase activities (responses) obtained from each experimental run and the predicted responses were much closer to each other. The maximum keratinase activity of 191 U/ml was recorded in 48 h with 3 g/l GFF concentration, 3% inoculum size, and at 35°C (run 17, Table 4). p-values for the model (< 0.0001) and for “lack of fit” (0.6271) indicated that the obtained experimental data were a good fit with the model (Table 5). Regression equation coefficients were calculated, and the data were fitted to a second-order polynomial equation, where A, B, and C represented pH, GFF concentration, and inoculum size (coded values), respectively. P-values of interactive terms (AB and BC) of the model indicated that high keratinase production depended on the interactions of the pair factors.
Table 4
CCD of RSM experiments using three independent parameters showing actual and predicted values of keratinase production
Table 5
ANOVA for response surface: quadratic model analysis of variance
RSM results showed that the obtained data fit well with the model. To measure the strength of the model, multiple correlation coefficients (R 2) and F-values were used. The higher accuracy of the model was indicated by the R 2 value of 0.9756, an adjusted R 2 value of 0.9537, and a high F value of 44.47. The closeness of R 2 and adjusted R 2 values to each other and to 1 further implied that only 2.19% of the total variation was not explained by the model and that there was a good correlation between the observed and predicted values. A 3D interactive plot showing keratinase activities with respect to pH and amount of GFFs is depicted Figure 3. The interaction response of inoculum and temperature showed a curvature, which suggests that keratinase activities were maximum at the middle points (3% inoculum size and 35°C temperature) – Figure 3A; however, keratinase as a function of the inoculum size and GFF concentration (Fig. 3B) showed that higher keratinase yield was achieved at the inoculum size of 3% and GFF concentration of 4 g/l. The maximum keratinase yield of 184 U/ml was achieved from the following optimum conditions: GFF concentrations (4.0 g/l), temperature (35°C), and inoculum size (3%). The data predicted from the model were verified by experiments performed in triplicate, and the average keratinase produced (182.5 U/ml) matched the predicted yield of 184.1 U/ml.
Degradation of heavy-metal-polluted GFFs by gellan-gum-immobilized GFF-DB
Cu- and Zn-polluted GFFs were degraded by gellangum-immobilized GFF-DB under optimized conditions, and the rate of degradation and the amount of heavy metal residue in the hydrolysates were compared with those of free-living GFF-DB. Immobilization of bacterial whole cells or their enzymes is a novel technique used in bioprocess and possesses various advantages such as stability, high efficiency, and reusability over the use of free cells (Ahmad et al., 2012). Even though previous studies involving melanized feathers have reported keratinase activities by immobilized bacteria (Yusuf et al., 2019), the present study was the first immobilization study involving GFFs. Immobilization of IY-BUK1 cells resulted in higher keratinase production and faster degradation of heavy-metal-polluted GFFs than that of free-living cells. Complete degradation of 5 g/l of polluted GFFs was achieved within 72 h with a higher amount of keratinase (214 U/ml) when 250 beads of immobilized GFF-DB were used as against 120 h and 191 U/ml when 3.5% of free cells were used (Table 6). A significant difference (P < 0.05) in the degradation of heavy-metalladen GFFs was observed between free and immobilized IY-BUK1. Key metabolic features of the immobilized cells, such as cell growth, might have been modified by the faster degradation of Cu- and Zn-laden GFFs (Moslemy et al., 2002). The residual Cu and Zn ions in the resultant protein hydrolysate produced by immobilized cells were analyzed and compared with those of free cells. The amount of residual Cu and Zn in the hydrolysates after complete degradation of Cu- and Zn-polluted GFFs was significantly lower than that of free cells, which agrees with the strain’s capacity to accumulate Cd and Pb following the degradation of melanized feathers (Abba et al., 2020). These findings indicate that Pseudochrobactrum sp. IY-BUK1 possesses the additional capacity to absorb/accumulate Cu and Zn besides featherdegrading properties.
Table 6
Average degradation of GFFs laden with Cu and Zn and their combination by immobilized IY-BUK1 strain compared with free cells
Similarly, the immobilized IY-BUK1 maintained the degradation of 5 g/l of different heavy-metal-laden GFFs for seven cycles of 72 h. The number of cycles decreased to four when the concentration of Cu and Zn adsorbed onto the GFFs increased to 33.2 ppm. This result is consistent with an earlier report in which immobilized Alcaligenes sp. AQ05-001 degraded feathers in FMB containing a mixture of Ag, Co, and Cu for eight cycles (Yusuf et al., 2019).
Conclusions
The results of this study revealed that GFFs possess the potential for adsorbing or removing Cu and Zn ions in aqueous solutions, and the potential improved further when pretreated with acetone and NaOH. Biodegradation of Cu- and Zn-laden GFFs was carried out successfully using Pseudochrobactrum sp. IYBUK-1 under optimized conditions. Immobilization of the GFFs degrading strain enhanced the degradation rate with a minimal concentration of Cu and Zn in protein hydrolysate produced. Thus, GFFs can serve as good biosorbents and, in combination with IY-BUK1, can be an alternative approach to an unfriendly way of disposing GFFs, thus preventing environmental pollution through bioremediation.