Introduction
Cannabidiol (CBD), commonly abbreviated as CBD, is a phytocannabinoid derived from Cannabis sativa and, in contrast to Δ-9-tetrahydrocannabinol (THC), it is non-psychotropic. CBD exhibits diverse properties, including neuroprotective, cardioprotective, and anti-inflammatory effects [1]. Currently, CBD products are utilized without regulatory approval, relying on perceived health benefits, although scientific evidence supporting these claims often lacks controlled clinical trials. The recognition of CBD’s ability to influence numerous biological targets forms the basis of these assertions. Notably, Epidiolex® is the sole product subjected to rigorous pharmacokinetic evaluation and clinical studies, receiving FDA approval as an over-the-counter drug for treating infantile-onset intractable seizures [1].
In this context, THC emerges as the primary psychoactive component of cannabis, responsible for the classic feeling of “high” or “euphoria”. Conversely, a substantial body of literature discusses CBD’s medical potential, particularly in addressing anxiolytic and antiepileptic effects. Popular sentiments also suggest additional therapeutic benefits, such as mitigating adverse side effects of THC, including intoxications, psychomotor dysfunctions, and psychotic manifestations [2].
Furthermore, the two main cannabinoid receptors, cannabinoid 1 (CB1) and cannabinoid 2 (CB2), are inhibitory G protein-coupled receptors consisting of a polypeptide chain with seven transmembrane segments that traverse the bilayer cell membrane lipid. CB1 receptors are predominantly found not only in the central nervous system [3] but also in various tissues, including the heart, lungs, intestines, kidneys, and liver [4]. On the other hand, CB2 receptors are localized in cells of the immune system [5], the gastrointestinal tract [6], and minimally in the central nervous system [7]. Specifically, CB2 receptors are identified in a particular type of the microglial cell in the human cerebellum, as confirmed by immunohistochemical analyses on tissue sections from the cerebellum’s white matter areas, demonstrating their presence in perivascular microglial cells [5].
THC functions as a partial agonist for both CBD receptors, while CBD inhibits metabolic enhancement by impeding the oxidation of THC to 11-OH THC. Acting as an inverse agonist of CB1, CBD limits its action. Additionally, CBD has the capacity to bind to CB2 as a low-affinity agonist and other non-cannabinoid receptors [8].
It is well established that THC primarily induces pharmacological effects such as analgesia, muscle relaxation, nausea reduction, appetite stimulation, and psychoactivity. In contrast, CBD has been demonstrated to possess neuroprotective, anticonvulsant, antipsychotic, muscle relaxant, anxiolytic, and antioxidant activities. Moreover, CBD diminishes both anxiety and the psychoactive effects of THC [9].
Preliminary pharmacological and behavioural activity tests affirm the similarity between endogenous anandamide, CB, and THC, all acting as partial agonists of the CB1 receptor. Conversely, CBD exhibits weak binding to CB1 and CB2 [9, 10]. However, CBD is portrayed as a neutral receptor antagonist, signifying its lack of direct activity on these receptors but its ability to reverse both agonist and inverse responses.
Moreover, CBD has demonstrated robust anti-inflammatory, immunomodulatory, and antioxidant properties in vitro [11]. Consequently, the therapeutic implications of CBD are multifaceted. Numerous studies detail its impact on neurological disorders, including Parkinson’s disease, Lennox-Gastaut syndrome, Huntington’s disease, and various forms of epilepsy [12, 13]. Research also extensively explores the effects of CBD on pain [14], inflammation [15], and the immune system [16].
In a noteworthy study, Palmieri et al. investigated the therapeutic effects of a CBD ointment administered on severe chronic skin diseases or resulting scars. The topical application of the CBD-enriched ointment significantly improved skin parameters, symptoms, and scores on specific questionnaires. Importantly, no irritant or allergic reactions were recorded during the treatment period. Consequently, the topical use of an ointment containing only CBD (without THC) has emerged as a safe and non-invasive alternative to enhance the quality of life for patients suffering from skin problems, particularly those associated with inflammation [17, 18].
In the context of dermatology, atopic dermatitis (or atopic eczema) stands out as a significant chronic inflammatory skin disease characterized by itching and skin manifestations, including erythema, desquamation, lichenification, and papulo-vesicular eruption. In some cases, it may be associated with asthma or allergic oculorhinitis. This condition predominantly affects children, adolescents, or adults and has multifactorial origins encompassing genetic, environmental, immunological, and psychological factors. Diagnosis primarily relies on clinical observations. Several reports have elucidated the involvement of T lymphocytes in the pathogenesis of atopic dermatitis [19].
For the treatment of mild forms, topical cortisone drugs and immunomodulatory topical drugs such as tacrolimus and pimecrolimus are commonly employed. Phototherapy is an option for extensive lesions, and antihistamines are widely used to control itching. In severe cases, systemic steroids and other immunosuppressants such as cyclosporine are utilized [20]. Recent therapeutic developments include monoclonal antibody-based therapies targeting interleukins pivotal in inflammation, such as dupilumab (anti IL-4 receptor α), tralokinumab (anti IL-13), or, in more resistant forms, Janus kinase inhibitors like baricitinib and upadacitinib.
Furthermore, CBD containing ointments represents a potential treatment option for atopic dermatitis. The increasing legalization of cannabis in numerous countries has opened up new research opportunities to explore the use of cannabinoids in topical formulations for therapeutic purposes.
Humans possess an endocannabinoid system that plays a pivotal role in regulating various physiological processes, encompassing the natural production of endocannabinoids and the proteins involved in their transport, synthesis, and degradation. The discovery of an endocannabinoid system in the skin, coupled with cannabinoids’ anti-inflammatory properties, has sparked interest in their application for treating inflammatory skin diseases [18].
Data compiled in the literature suggest that CBD functions as an immunomodulator, directly suppressing the activation of immune cells such as monocytes, macrophages, neutrophil granulocytes, dendritic cells, and T cells, inducing apoptosis, down-regulation, and promoting regulatory T cells instead [21]. Consequently, there is a significant suppression of production of inflammatory cytokines, including TNF-α, IFN-γ, IL-6, IL-1β, IL-2, IL-17A, and chemokines such as CCL-2.
Material and methods
Isolation of peripheral blood mononuclear cells, in vitro proliferation assay and cell viability assessment
Blood samples were collected from healthy donors, and peripheral blood mononuclear cells (PBMCs) were isolated through gradient centrifugation using Ficoll-hypaque (GE Healthcare, Milan, Italy). Subsequently, PBMCs were stained with carboxyfluorescein succinimidyl ester (CFSE; 5 μM) for 5 min, washed, and cultured in RPMI medium supplemented with 10% autologous serum.
PBMCs were either exposed to the mitogenic phytohemagglutinin A (PHA; 2 μg/ml) alone or in combination with CBD extract (Plant of Life, Italy), tested at three different doses (0.1 and 1 mg/ml), or to CBD alone (same two doses) in triplicate microcultures. Negative controls were established with no stimulus. After 5 days, cell viability was assessed by trypan blue exclusion. Subsequently, cells were harvested, washed, and stained for 20 mins with fluorochrome-coupled antibodies: CD3-APCeF780 and CD19-eF450 (eBioscience, ThermoFisher, Milan, Italy), distinguishing T and B cells, respectively.
Following the incubation period, cells were washed and analysed by flow cytometry using the Navios 3L 10C cytometer (Beckman Coulter, Milan, Italy) to detect proliferating T and B cells. Subsequent analysis was performed using FlowJo software (Tree Star Inc, OR, USA).
In vitro assessment of CBD’s cytotoxicity
The U937 cell line (ATCC) was cultured in vitro in RPMI medium supplemented with 10% FBS and plated without stimulation or with two different doses of CBD (0.1 and 1 mg/ml), all in triplicates, for 48 h. Following the respective incubation periods, cells were harvested, washed, and centrifuged onto microscope slides using a cytospin centrifuge. The slides were subsequently stained with the May-Grunwald and Giemsa protocol and examined utilizing the Nexcope microscope (Model NE620) along with 5MP camera software. Quantification of damaged cells was performed using Image J software, employing the multi-point tool. In particular, the cells showing morphological alteration such as shrinking of the cytoplasm, appearance of cytoplasmic vacuoles and nuclei fragmentation were considered as damaged.
Patients
Twenty-eight patients diagnosed with atopic dermatitis participated in our study, categorized into two groups. The first group, termed the “control group”, comprised 10 patients (9 females and 1 male; mean age: 48.6 ±17.7 years). This group received daily application of a topical 0.05% clobetasol ointment and, as an adjuvant treatment, utilized a cleansing oil (Bioderma, Italy) daily for 5 weeks. The second group, referred to as the “CBD group”, consisted of 18 patients (10 females, 8 males; mean age: 47.8 ±17.3 years). This group was provided with 0.05% clobetasol ointment and a CBD-based cleansing cream (containing a concentration of 0.05 mg/ml; The Allergist, Italy), which they applied daily for 5 weeks.
To monitor clinical symptoms, especially itch, patients utilized a Visual Analogue Scale (VAS) to record their experiences. Additionally, all patients received concurrent antihistamine treatment (fexofenadine 120 mg 1 pill/day for 20 days).
Results
CBD inhibits lymphocyte proliferation in a dose-dependent manner
We aimed to evaluate the effect of CBD on lymphocyte proliferation in vitro by conducting a flow cytometry-based proliferation assay using CFSE, as previously described [22]. PBMCs were isolated from 3 healthy donors (2 men, 1 woman; mean of age 36; with no clinical record of allergy, not taking steroids or antihistamine drugs), stained with CFSE, and cultured in the presence of the mitogenic PHA alone or in combination with CBD extract (Plant of Life, Italy) at two different doses (0.1 and 1 mg/ml) to assess the impact of CBD on lymphocyte proliferation induced by PHA. Controls included untreated PBMCs as well as CBD alone (same doses). After incubation, cells were harvested, stained with fluorochrome-coupled antibodies to distinguish T and B cells (Figure 1 A), and analysed by flow cytometry. An example of T cell proliferation under the different conditions is shown in Figure 1 B. The baseline proliferation rate of T cells and B cells in untreated cultures was 0.707 ±0.314% and 4.427 ±3.239%, respectively. The PHA-induced proliferation rate was 44 ±24% for T cells and 35.95 ±26.19% for B cells (Figure 1 C). In Figure 1 D the graph illustrates the fold change in T and B cell proliferation rates when treated with CBD and PHA compared to PHA alone.
Figure 1
Freshly isolated PBMCs were stained with CFSE and cultured with PHA alone or in combination with CBD, tested at 2 different doses. Untreated cultures and cells treated with CBD alone were used as controls. After culture, cells were harvested and stained with anti-CD3 and anti-CD19 antibodies and analysed by flow cytometry. After gating on total lymphocytes, B and T cells were selected (A), and the percentage of proliferating T cells and B cells (B) were then measured for each condition. Representative dot plots of proliferating T cells from one donor are shown (A). The stacked overlays of CFSE expression in T cells (left panel) and B cells (right panel) for all conditions are shown for one donor (B). Proliferation rates of T (light grey) and B (dark grey) cells are presented as mean ± SD (C). The graph illustrates the fold change of T and B cell proliferation rate in (CBD + PHA) with respect to PHA alone (D). Cell viability assessment as revealed by trypan blue exclusion (E)
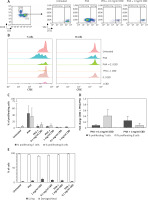
Interestingly, CBD addition reduced the percentages of proliferating T and B cells in a dose-dependent manner. The proliferation rate with the 0.1 mg/ml of CBD was 5.31 ±4.46% and 9.39 ±6.86% for T and B cells, respectively; 4.68 ±0.87% and 6.67 ±6.67% with 1 mg/ml of CBD. Importantly, the cell viability assessment was > 95% in all the tested conditions.
Effect of CBD on promyelocyte cell line
Next, we assessed the cytotoxic effect of CBD on cell cultures. We tested the same doses of CBD tested in the PBMC experiments (0.1 and 1 mg/ml) on U937 cell lines for 24 h and 48 h. We assessed the morphological changes of U937 cells, a promonocytic cell line, as described above (see material and methods section). Interestingly U937 cells were cultured alone or with CBD at 0.1 mg/ml; the tested doses did not show significant morphological changes in U937 cells. The microscopy analysis of the cells cultured with 1 mg/ml of CBD revealed formation of cytoplasmic vacuoles (Figure 2 A). Thus, we calculated the percentage of living and damaged cells using Image J software (Figure 2 B). As shown in Figure 2 C, incubation of U937 cells with 1 mg/ml of CBD induced an increase in damaged cell quantification.
Figure 2
U 937 cells (promonocytic cell line) were cultured alone or with increasing doses of CBD for 48 h, harvested, spun on microscope slides, and stained with the May-Grunwald and Giemsa method (A). Untreated cells, cells (CTRL)/control cells or those treated with 0.1 and 1 mg/ml CBD (from the upper left panel, clockwise). Methodology used for the quantification of damaged cells (B). Quantification of damaged cells (C). Clinical efficacy of the CBD-containing preparation (cleansing cream) was observed, with a statistically significant decrease noted in the CBD-treated group compared to the control group over a 5-week period (D)
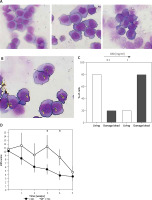
In vivo efficacy of CBD-adjuvant treatment
As shown in Figure 2 D, the treatment with clobetasol 0.05% ointment and cleansing oil (control group) induced a reduction in the VAS score after 5 weeks of treatment. Interestingly, the adjuvant treatment with CBD-enriched cleansing cream induced a steady decline of VAS score starting from the first week (Figure 2 D).
Discussion
CBD has demonstrated multiple therapeutic properties, including neuroprotective, anticonvulsant, antipsychotic, muscle relaxant, anxiolytic, and antioxidant activity. Additionally, CBD has been shown to reduce anxiety and the psychoactive effects of THC. The mechanisms of action of CBD involve interaction with cannabinoid receptors, CB1 and CB2, as well as other non-cannabinoid receptors.
Our research examined the effects of CBD on lymphocyte proliferation in vitro and evaluated its cytotoxicity on a cell line. Our results clearly indicate that CBD inhibits lymphocyte proliferation. Indeed, the addition of CBD to PHA mitogen-stimulated lymphocyte cultures significantly reduced the percentage of proliferating T and B lymphocytes. These data support the anti-inflammatory effect of CBD on lymphocytes. CBD appears to suppress the activation of immune system cells, such as monocytes, macrophages, neutrophil granulocytes, dendritic cells, and lymphocytes, and promote the action of immunoregulatory cells [10, 23, 24]. This immunomodulatory effect could contribute to the therapeutic benefits of CBD in the management of inflammatory skin disorders such as atopic dermatitis.
It is important to note that CBD has shown no cytotoxic effects at the tested doses on human PBMCs.
Likewise, the incubation of U937 cells with CBD doses did not exert a cytotoxic effect at the 0.1 mg/ml dose. On the other hand, at the 1 mg/ml dose, phenotypical findings of cell damage were observed. CBD is currently used for both pharmacological and cosmetic purposes. Our data suggest that, in the field of cosmetics, a concentration of CBD lower than 1 mg/ml should be used. Indeed, according to our preliminary results, the use of an adjuvant treatment with CBD-containing cleansing cream at the dose of 0.05 mg/ml is useful and well tolerated in the treatment of itch induced by atopic dermatitis.
Conclusions
Our results have highlighted the anti-inflammatory and immunomodulatory effect of CBD, support the inhibitory effect of CBD on lymphocyte proliferation, and suggest its potential as a therapeutic agent for inflammatory skin disorders.
However, further research is needed to determine the optimal doses and methods of administration of CBD as well as to evaluate its long-term safety and efficacy in patients suffering from atopic dermatitis and other inflammatory dermatological skin conditions.








