Introduction
Taurine (β-amino-ethane sulfonic acid; TAU) is the most plentiful free amino acid in the human body [1, 2]. This amino acid is abundantly found in almost all cell types [1, 2]. Although TAU does not contribute to the protein’s structure, several physiological roles have been attributed to this amino acid [1, 2]. TAU plays a significant role in bile acid conjugation and acts as an essential osmolyte in various biological systems [1-5]. Despite the ubiquitous physiological functions of TAU, its action in the pathophysiology of diseases is largely disputed. On the other hand, it has been found that TAU deficiency can seriously impair the function of several organs such as the skeletal muscle and heart [6-9]. Therefore, it is essential to investigate the role of TAU deficiency in the pathogenesis of the human disease.
Body TAU can be synthesized in the liver or supplied from the diet. The source of body TAU is essentially species-dependent [10, 11]. Some species, such as foxes and felines, entirely depend on the dietary TAU [10, 11]. In humans, the hepatic TAU synthesizing capability is negligible, and this compound is primarily supplied from the diet [12, 13]. Seafood (e.g., oysters and muscles) is a well-known dietary source of TAU [14]. TAU is taken up by various cell types via specific transporters (TauT) [15]. The accumulation of TAU in different organs is widely variable [15]. Tissues such as the heart and skeletal muscle contain very high concentrations of TAU [15]. At the subcellular levels, TAU is mostly compartmentalized in mitochondria [12, 16]. Several lines of evidence support the pivotal role of TAU in mitochondrial function [17-20]. It has been found that TAU can regulate mitochondrial energy metabolism, attenuate mitochondria-facilitated reactive oxygen species (ROS) formation, and prevent mitochondria-mediated apoptosis and cell death [17-24]. Interestingly, it has been found that TAU is also incorporated in mitochondrial tRNA structure [12, 17, 25-27]. Thus, the synthesis of mitochondrial proteins (e.g., respiratory chain complexes) is impaired in TAU deficiency [12, 17, 25-27]. Decreased integrity of mitochondrial respiratory chain complexes is repeatedly mentioned in TAU deficiency conditions [22, 28]. These events may impair ATP metabolism, cellular energy crisis, cell death, and organ injury [22, 28].
Cholestasis is a severe clinical complication induced by xenobiotics or liver disease [29, 30]. The liver is the main organ influenced by cholestasis [31-34]. Prolonged cholestasis can lead to tissue fibrosis, cirrhosis, and fulminant hepatic failure [29, 30]. However, organs other than the liver may also be affected during cholestasis [31-33]. It is well known that cholestatic liver disease is related to brain injury, skeletal muscle damage, cardiovascular dysfunction, lung injury, renal impairment, intestinal damage and permeability, and poor function of reproductive organs [35-42]. Several lines of evidence indicate that bile duct ligation (BDL) is a suitable experimental tool to investigate cholestasis-induced organ injury [33, 43-48]. Severe liver histopathological changes, cardiac dysfunction, skeletal muscle atrophy, muscle mass loss (sarcopenia), brain and lung injury, hepatic encephalopathy, intestinal and renal damage (cholemic nephropathy), and poor reproductive organs function are appropriately induced in the BDL model of cholestasis [33, 36, 42-47]. On the other hand, various investigations, including our research on BDL animals, indicate the essential role of mitochondrial impairment in the pathogenesis of cholestasis-associated complications [23, 33, 38, 41, 49-66].
Based on the above literature, the current investigation was designed to evaluate tissue and mitochondrial TAU levels in various organs of BDL rats. The obtained data could help to identify factors involved in the pathogenesis of cholestasis/cirrhosis-induced organ injury and, eventually, the development of therapeutic options in this disease.
Material and methods
Reagents
Trichloroacetic acid, potassium chloride, sucrose, 3-(N-morpholino) propanesulfonic acid (MOPS), iodoacetic acid, ethylenediamine tetra-acetic acid (EDTA), potassium hydroxide, phosphoric acid, acetonitrile HPLC grade, 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethane sulfonic acid (HEPES), sodium chloride, 2amino2-hydroxymethyl-propane-1,3-diol-hydrochloride (Tris-HCl), sucrose, trypsin, bovine serum albumin (BSA), trichloroacetic acid, sodium carbonate, and D-mannitol were obtained from Merck (Darmstadt, Germany). Ketamine and xylazine were purchased from Bioveta (Czech Republic). Anhydride calcium, methyl tetrazolium, dinitrofluorobenzene (DNFB), dimethyl sulfoxide, and taurine (2-aminoethanesulfonic acid) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Animals
Forty-eight healthy mature male and female Sprague Dawley (SD) rats (250-300 g) were obtained from Shiraz University of Medical Sciences, Shiraz, Iran. Animals were housed in polystyrene cages over wood-chip bedding. There was an environmental temperature of 24 ±1°C and a 12 l photoschedule, along with ≈ 40% relative humidity. Rats had free access to a regular rodents’ chow diet (RoyanFeed, Isfahan, Iran) and tap water [67]. An ethics committee approved all animal experiments in Shiraz University of Medical Sciences, Shiraz, Iran (#95-01-36-11587) and the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments).
Experimental setup
Bile duct ligation is an appropriate animal model to investigate the adverse effect of cholestasis in the liver [42, 62, 68-70]. For BDL surgery, rats were anesthetized (a mixture of 8 mg/kg of xylazine and 60 mg/kg of ketamine, intraperitoneally [IP]). Then, a midline incision through the linea alba was made, and the common bile duct was localized and doubly ligated [42, 62, 68-70]. The sham operation involved laparotomy and bile duct localization without ligation.
Sample collection
Eight animals per group (sham-operated or BDL animals) were anesthetized (thiopental 80 mg/kg) at 14 and 42 days after the BDL operation. Tissue samples including brain, heart, liver, kidney, skeletal muscle, lung, intestine, ovary, testis, and blood samples were collected. Equal amounts of tissue samples (5% w : v) were homogenized in a solution containing 70 mM mannitol, 220 mM sucrose, 2 mM HEPES, 0.5 mM EGTA, and 0.1% essentially fatty acid-free bovine serum albumin (pH = 7.4) [63]. One milliliter of each blood sample was centrifuged (4000 g, 15 min, 4°C) and used for serum biochemical analysis. One milliliter of tissue homogenate was used for TAU evaluation, and the rest of the samples were used for mitochondria isolation. Liver tissue samples were also histopathologically analyzed (H&E staining for regular histopathological alterations and trichrome staining for tissue fibrosis) to confirm the occurrence of cholestasis/cirrhosis in the current model.
Mitochondria isolation protocol
Mitochondria were isolated from different tissues with high mitochondrial content (brain, heart, liver, skeletal muscle, and kidney) based on the differential centrifugation protocol [33, 71-75]. The right and left hind legs’ gastrocnemius muscle was used for skeletal muscle mitochondria isolation. Samples of heart tissue and skeletal muscle were minced in isolation buffer (70 mM mannitol, 220 mM sucrose, 2 mM HEPES, 0.5 mM EGTA, and 0.1% essentially fatty acid-free bovine serum albumin, pH = 7.4) containing trypsin (0.1% w : v), and incubated on ice for 15 minutes [38, 71].Then, samples were centrifuged (10,000 g, 10 min, 4°C), and the supernatant was discarded. The pellet (heart and skeletal muscle tissue) was homogenized in the isolation buffer at a 10 : 1 ratio of isolation buffer to tissue (v : w) and homogenized [71, 76, 77]. Other tissues were washed and minced in an ice-cold (4°C) isolation buffer medium. The minced tissues were transported into a fresh buffer (10 : 1 ratio) medium and homogenized. Then, the mitochondria-rich fraction was isolated by the differential centrifugation method [53, 67, 71, 78, 79]. First, tissue sample homogenates were centrifuged at 1000 g for 20 min (4°C) to pellet intact cells and RBCs. Then, the supernatant was centrifuged (10,000 g, 4°C, 20 min) to pellet the mitochondrial fraction. The crude mitochondrial fraction was further centrifuged at least three times (12,000 g, 4°C, 20 min) [80, 81]. Protein levels were measured using bovine serum albumin as a standard based on the Bradford method.
Tissue and mitochondrial taurine content
Samples (1 ml of the 5 mg protein/ml of isolated mitochondrial preparations or 1 ml of 10% w : v of tissue homogenate) were treated with 100 µl of TCA (50% w : v), vortexed well (30 s), and incubated at room temperature for 10 minutes. Afterward, tubes were vortexed again and centrifuged (15,000 g, 20 min). Then, the supernatant was collected in 10 ml tubes and treated with 2 ml of carbonate buffer (0.1 M, pH = 9.0), 0.5 ml of DMSO, and 100 µl of DNFB. Samples were protected from light and mixed well (vortexed for 30 s) then incubated at 40°C for 15 min. After the incubation period, samples were centrifuged (16,000 g, 20 min) and protected from light. Samples (25 µl) were injected into an HPLC apparatus consisting of a C18 column (250 × 4.6 mm, Alltech Econosphere, 3 µm) and a UV detector (set at λ = 360 nm) [82]. Mobile phases were composed of buffer A (phosphate buffer, 0.01 M, pH = 3.0) and buffer B (HPLC grade acetonitrile). The gradient program begins at 10% B, ramps to 25% Bat 10 minutes, then ramps to 50% B at 15 minutes, and was held at 50% B until 20 minutes. Next, the flow was back to 10% buffer B until the run time ended (30 min). The flow rate was 1 ml/min [82].
Results
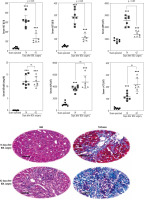
Assessment of serum biomarkers revealed a significant increase in alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) in BDL animals compared with the sham-operated group (Fig. 1). Moreover, serum bilirubin, alkaline phosphatase (ALP), and γ-glutamyltranspeptidase (γ-GT) drastically increased at different time intervals after the BDL operation, although there was no significant difference between 14- and 42-day groups. It should be mentioned that the level of biomarkers such as ALP, γ-GT, and bilirubin is constantly high in the BDL model of cholestasis due to permanent ligation of the bile duct. Furthermore, tissue histopathological alterations, including bile duct proliferation, necrosis, and inflammatory cell infiltration, as well as significant collagen deposition (trichrome stain), were detected in the liver of BDL animals (Fig. 1). These data indicate the appropriate induction of cholestasis/cirrhosis in the current study.
Fig. 1
Liver tissue histopathological alterations and serum biomarkers indicate appropriate induction of cholestasis/cirrhosis in bile duct ligated (BDL) rats. Bile duct proliferation and inflammatory cell infiltration were detected in the liver of cholestatic animals (H&E stain). Scale bar = 100 µm. Moreover, significant collagen deposition (blue area in Masson-trichrome stain) indicates liver fibrotic areas. Data are presented as mean ± SD (n = 7). ***Indicates significantly different from the sham-operated group (p < 0.001). ns – not significant
TAU levels were assessed in various tissues of cholestatic rats 14 and 42 days after the BDL operation. Significant depletion in TAU content was evident in most tissues 14 days after BDL. On the other hand, the TAU content of all tissues was drastically decreased 42 days after the BDL surgery. As TAU depletion was not significant at 14 days after BDL operation, this parameter was not time-dependent in tissues such as the liver, ovary, lung, heart, and colon (Fig. 2).
Fig. 2
Tissue taurine levels in bile duct ligated (BDL) rats. Data are shown as box and whiskers (min to max). Asterisks indicate significantly different as compared with the sham-operated group (*p < 0.05 and ***p < 0.001). ns – not significant
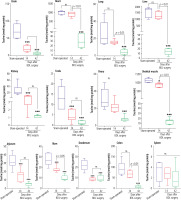
Mitochondrial TAU content of tissues, including skeletal muscle, brain, heart, liver, and kidney, significantly decreased at both intervals of 14 and 42 days after the BDL operation. The decrease in TAU levels of mitochondria was not time-dependent in most tissues (heart, brain, and kidney) assessed in the current study (Fig. 3).
Discussion
Taurine is a very safe amino acid abundantly found in the human body. Various experimental models have highlighted the physiological and pharmacological roles for TAU [1, 2]. However, the role of TAU deficiency in the pathogenesis of many human diseases is far from clear. The data obtained from the current investigation revealed a significant decrease in TAU content of several tissues as well as mitochondria in BDL rats as a reliable animal model of cholestasis/cirrhosis. These findings indicate a pivotal role for tissue and mitochondrial TAU in the pathogenesis of cholestasis/cirrhosis-induced organ injury. As TAU plays a viable function as an osmolyte in various tissues [1, 2], and most importantly is crucial for mitochondrial function and energy metabolism [12, 17, 22, 25-28], significant alteration in its levels could play pathogenic roles in cholestasis/cirrhosis-linked complications.
The effects of TAU on mitochondrial indices are an exciting feature of this amino acid [12, 17, 25-27]. TAU is localized in mitochondria via specific transporters [83, 84]. Moreover, some studies also found that TAU could be synthesized in the mitochondrial matrix [85]. These data indicate the importance of TAU in mitochondrial function. Therefore, disruption of the fundamental processes such as energy metabolism could accompany cellular TAU depletion. Based on these data, investigating the TAU level in cholestasis/cirrhosis could enhance our understanding of the mechanism of organ injury in these pathological conditions and provide viable therapeutic options.
Oxidative stress is a well-known phenomenon in various tissues of cholestatic animals [34, 62, 86-89]. Oxidative stress could damage multiple cellular targets, including proteins, lipids, and essential organelles such as mitochondria [31, 35, 88, 90-97]. It is well known that oxidative stress is a general phenomenon in cholestasis [34, 87, 88]. On the other hand, mitochondria are the major sources of intracellular ROS [98]. Interestingly, various investigations have mentioned the role of TAU in preventing mitochondria-facilitated ROS formation and oxidative stress [19, 20, 22]. The role of TAU in mitochondrial tRNA structure and function is an interesting feature of this amino acid [15, 26, 83, 99-102]. It is well known that the proper modification of mitochondrial tRNA structure by TAU leads to appropriate synthesis and function of mitochondrial proteins (e.g., mitochondrial respiratory chain complexes) [15, 26, 83, 99-102]. Thus, the impact of TAU in the appropriate synthesis and function of mitochondria respiratory chain complexes is a fundamental role of this compound in preventing mitochondria-mediated oxidative stress [19, 20]. In our recent studies on cholestatic animals, we repeatedly found that TAU supplementation could improve mitochondrial function and blunt oxidative stress in various organs [23, 103, 104]. Based on these data, TAU depletion in various organs during cholestasis/cirrhosis is directly connected to the occurrence of oxidative stress and its linked complications.
Some studies indicate that TAU deficiency mediates apoptosis and cell death through a mitochondria-dependent pathway [28]. Interestingly, it has been found that TAU deficiency could lead to the induction of mitochondrial permeability transition pore (mPT) [105]. Jong et al. revealed that mitochondrial TAU content is directly associated with increased cell apoptosis [105]. mPT induction could cause the release of cell death mediators (e.g., cytochrome c) from this organelle [105]. The release of other cell death mediators such as apoptosis-inducing factor (AIF) also has been reported in different tissues of cholestatic animals [53]. However, more studies are needed to identify a connection between mitochondrial TAU deficiency and the release of such mediators from mitochondria.
It has been well documented that collagen deposition and fibrosis are important events in the liver, kidney, lung, and heart during cholestasis/cirrhosis [47, 106-110]. Tissue fibrosis results from a complex process connected to oxidative stress [111]. On the other hand, mitochondria are significant sources of intracellular ROS [98]. Therefore, mitochondrial impairment could play a pathogenic role in tissue fibrosis during cholestasis. It has been reported that protecting cellular mitochondria could significantly prevent liver injury and fibrosis in an experimental model of cirrhosis [112]. Pérez et al. found that mitochondria-mediated cell death could play a crucial role in liver injury during cirrhosis [112]. These investigations highlight the importance of mitochondrial impairment in chronic liver disease, leading to fibrotic lesions. The antifibrotic properties of TAU have also been frequently mentioned in various tissues [113-117]. Previous investigations indicated that liver mitochondrial function was severely impaired in the BDL model of hepatic fibrosis [33, 118-120]. On the other hand, it has been found that TAU could significantly enhance mitochondrial function and prevent mitochondria-facilitated ROS formation and oxidative stress [103, 121]. Therefore, we might be able to hypothesize that a part of the antifibrotic effects of TAU could be mediated through mitochondrial-dependent mechanisms.
In the following parts, the role of TAU deficiency and its potential link with organ injury reported in cholestasis/cirrhosis (liver, brain, heart, kidney, skeletal muscle, and intestinal damage) is discussed in the context of the fundamental role of this amino acid in mitochondrial function and mitigating oxidative stress.
It has been found that TAU had significant hepatoprotective properties in both experimental models and human cases of cholestasis/cirrhosis [122-124]. For example, the effect of TAU on portal hypertension is an exciting feature of this amino acid [122-124]. The impacts of TAU on the morphology of the liver and biomechanical properties of the portal vein seem to be involved in its effects on portal hypertension in cirrhotic patients [122-124]. It is well known that the effects of TAU in mitigating oxidative stress and its associated complications in the liver play a pivotal role in its hepatoprotective properties [70, 103, 121, 125-135]. On the other hand, the effects of TAU on hepatocytes’ mitochondrial function have also been repeatedly investigated [33, 103, 118-121, 136-138]. It is well established that TAU could enhance mitochondria energy metabolism, prevent mitochondrial permeabilization, and blunt mitochondria-mediated cell death in experimental models of hepatic injury [33, 103, 118-121, 136, 137]. These data indicate that TAU is an excellent and safe compound for managing liver dysfunction as the main complication in cholestasis/cirrhosis. The current study found that liver tissue and hepatocytes’ mitochondrial TAU content were significantly depleted in cholestatic/cirrhotic animals (Figs. 2 and 3). Therefore, TAU supplementation could be a viable therapeutic option to blunt liver injury during cholestasis/cirrhosis.
Brain injury is a critical complication in cholestasis/cirrhosis [139, 140]. Several mechanisms have been proposed for cholestasis/cirrhosis-induced brain injury [139-142]. The first, and probably the most important one, is the disruption of the urea cycle in the liver and the accumulation of ammonia in plasma and brain tissue [139]. Ammonia is a neurotoxin, and it has been repeatedly mentioned that this agent is responsible for neuronal damage, cognitive dysfunction, brain edema, and coma in cirrhotic patients [139]. Some other compounds, such as bilirubin and manganese (Mn), also accumulate in different tissues such as the brain during cholestasis [139]. The mentioned compounds are well-known neurotoxicants [139]. Oxidative stress is a common feature of cholestasis/cirrhosis-induced brain injury [139, 143, 144]. Also, mitochondrial injury seems to play a crucial role in the pathogenesis of these complications [139]. All bile constituents such as Mn, bilirubin, and bile acids have adverse effects on mitochondrial function and energy metabolism [139, 145, 146]. In the current study, we discovered a significant decline in the TAU content of the brain tissue and isolated mitochondria (Figs. 2 and 3). On the other hand, a plethora of evidence indicates that this amino acid dramatically alleviates oxidative stress and enhances mitochondrial function in various experimental models of brain injury [147-149]. Our previous studies also found that TAU could improve brain mitochondrial function, mitigate biomarkers of oxidative stress, and enhance animals’ locomotor activity [70, 76, 103]. These data suggest that cholestatic/cirrhotic patients could benefit from the neuroprotective properties of TAU.
Sarcopenia or muscle mass loss is a serious complication in cirrhotic patients, leading to severe disability [51, 150-152]. Cirrhosis-induced sarcopenia could also significantly influence the outcome of therapeutic interventions such as liver transplantation [150, 153]. Several studies have mentioned that mitochondrial impairment and oxidative stress are crucial mechanisms involved in the pathogenesis of sarcopenia-induced muscle injury [51]. The effects of TAU on muscle function are another critical feature of this amino acid [24, 154-158]. TAU is found at very high concentrations in the skeletal muscle [155, 157]. It has been found that the effect of TAU on mitochondrial function and energy metabolism is an essential feature of this amino acid in the skeletal muscle [9, 20, 24, 159]. Therefore, decreased muscle TAU level is associated with muscle dysfunction [9, 24]. Some studies have revealed that TAU transporter knock-out animals exhibited a significant decrease in skeletal muscle mass and function [9, 160]. Interestingly, changes in mitochondrial morphology of the skeletal muscle have also been detected in the ultrastructural analysis of the tissue samples from TAU deficient models [8, 9]. On the other hand, several studies indicate that TAU could significantly enhance muscle performance [24, 69, 161-165]. These events indicate that TAU plays a crucial role in skeletal muscle function. A big part of TAU effects on the skeletal muscle is mediated through this amino acid’s impact on mitochondrial function and energy metabolism [24, 69, 161, 165]. In the current study, we detected that TAU levels in skeletal muscle tissue and mitochondria were significantly decreased at different time intervals after BDL surgery (Figs. 2 and 3); thus, TAU deficiency-associated mitochondrial impairment could play a critical role in muscle injury induced by cholestasis/cirrhosis. Therefore, the administration of this amino acid could be a viable strategy for managing cirrhosis-associated muscle dysfunction and sarcopenia.
Cardiac contraction abnormalities, decreased cardiac output, and heart failure could occur in cholestasis/cirrhosis [166-170]. Cardiac arrhythmia is also a com-mon pathological finding in cirrhotic patients [171, 172]. Previous studies also indicate oxidative stress, inflammation, and mitochondrial impairment in the heart tissue during cirrhosis [23, 173-175]. On the other hand, several studies suggest that TAU deficiency is associated with cardiac abnormalities [7, 176]. It has been found that pathological conditions such as cardiac atrophy and heart failure accompanied TAU deficiency [7, 176]. Recent studies also mentioned that essential pathways involved in tissue energy metabolism, such as fatty acid oxidation, are suppressed in the cardiac muscle under TAU deficiency conditions [177]. Interestingly, in TAU transporter knockout models, ultrastructural changes in cardiomyocytes’ mitochondria have been detected [7]. Recently we found that TAU could significantly improve mitochondrial function and blunted oxidative stress in the heart tissue of cirrhotic animals [178]. Moreover, it has been repeatedly reported that TAU could normalize various types of arrhythmia [179]. The effects of TAU on cirrhosis-induced arrhythmia could be an interesting subject for future investigations. The current study detected that TAU levels dropped significantly in cardiac tissue and isolated mitochondria (Figs. 2 and 3). All these data support the positive impact of TAU on cardiac function. Therefore, compensating for TAU deficiency could play an essential role in blunting adverse cardiac events in cholestatic/cirrhotic patients.
The adverse effect of cholestasis/cirrhosis on renal function is another essential subject widely investigated [93, 180-184]. At the early stages of bile duct obstruction, several potentially cytotoxic molecules such as bilirubin and bile acids are suspected to be responsible for direct renal injury [38, 71, 184]. Cholemic nephropathy is a phenomenon developed as an early response to cholestasis [38]. Bile cast formation, tubular damage, tissue necrosis, and inflammatory cell infiltration have been detected in cholemic nephropathy [38, 45, 181]. Renal fibrosis could also occur in cholestasis [38]. On the other hand, cirrhosis-induced renal injury is mainly developed as a hepatorenal syndrome [185]. In addition to histopathological changes detected in cholemic nephropathy, progressive tissue fibrosis, severe alteration in renal vasculature, hemodynamic changes, and renal failure are common in hepatorenal syndrome [185]. Mechanistically, oxidative stress and its related events seem to play a fundamental role in the pathogenesis of cholemic nephropathy and hepatorenal syndrome [38, 71, 90, 186, 187]. Additionally, several investigations have revealed the importance of mitochondrial impairment in the mechanism of cholestasis-induced renal injury [60, 188]. Mitochondrial impairment in the kidney could lead to an energy crisis and subsequent impairment of high energy demand processes such as reabsorption of chemicals in the renal tubules [189, 190]. This phenomenon is known as the Fanconi syndrome [90]. There are reports of the Fanconi syndrome linked with cholestasis/cirrhosis [92, 191-193]. Several studies suggested the therapeutic role of antioxidants against cholestasis-induced renal injury [39, 125, 194, 195]. Recently, we found that TAU administration to cholestatic rats could significantly alleviate cholemic nephropathy in BDL animals [104]. The effects of TAU on mitochondrial parameters and oxidative stress seem to be the primary mechanism for its renoprotective properties in cholestatic rats [104]. On the other hand, the effects of TAU on the renal blood flow, osmoregulation, glomeruli filtration rate, and ion absorption and secretion are mentioned as the physiological roles of this amino acid in the renal system [196, 197]. Several studies have revealed the positive effects of TAU on various types of renal disorders and xenobiotics-induced renal injury [126, 127, 197-204]. In the current study, we found that tissue and mitochondrial TAU levels were dramatically decreased in the kidney of BDL rats (Figs. 2 and 3). Therefore, TAU deficiency could play a significant role in the pathogenesis of oxidative stress, mitochondrial dysfunction, and renal injury in cholestasis/cirrhosis. Based on these data, TAU supplementation could serve as a therapeutic option to mitigate cholestasis/cirrhosis-linked renal impairment.
The role of TAU in reproductive system function, in both males and females, is also the subject of many investigations in this field [205-214]. On the other hand, it has been found that reproductive organs are severely damaged during cholestasis [59]. Oxidative stress and mitochondrial impairment seem to play a pivotal role in the mechanisms of reproductive system injury in cholestasis [59]. Meanwhile, it is well established that the effects of TAU in counteracting oxidative stress and its related complications are a major mechanism for the protective properties of this amino acid in reproductive organs [214, 215]. It has also been reported that TAU could significantly improve parameters such as sperm motility, sperm antioxidant levels, ATP content, and sperm capacitation [205, 213, 216-220]. TAU also could regulate the synthesis and release of important hormones such as testosterone and luteinizing hormone (LH) [213]. All these data highlight the crucial role of TAU in the reproductive system. In the current study, we found that testis and ovary TAU levels were significantly depleted in cholestatic rats (Fig. 2). Therefore, TAU depletion could be linked with poor reproductive factors in males and females. In this context, TAU supplementation could be considered as a strategy to protect reproductive organs in cholestasis/cirrhosis.
Several parameters could be involved in the mechanism of tissue and mitochondrial TAU depletion in cholestasis/cirrhosis. First, and most notably in the current model, the TAU synthesis is disrupted in the liver during cholestasis due to severe hepatic injury (Fig. 4). This could be the leading cause of perturbed TAU levels in rodent experimental models, as the liver is the main organ synthesizing this amino acid. However, humans have relied on dietary TAU, and the amount of TAU synthesis in the liver is negligible. Therefore, other factors such as disturbed absorbance of this amino acid could also lower TAU levels in cholestasis/cirrhosis. In the current study, the intestinal (duodenum, ileum, and jejunum) TAU level was significantly depleted in BDL rats. It is well known that TAU is a vital osmolyte that preserves enterocyte integrity [221, 222]. Hence, a depleted TAU level in the intestine could disturb the absorbance of many nutrients, including TAU itself (Fig. 4). These data indicate that TAU supplementation could protect intestinal tissue and prevent disturbances in the process of absorption of vital compounds into the bloodstream. On the other hand, these data suggest that future studies on the clinical administration of TAU to cholestatic/cirrhotic patients should be through the parenteral route because of intestinal damage in these patients.
Fig. 4
Taurine is synthesized in the liver of some species, such as rats and dogs, by utilizing methionine and cysteine amino acids. Some species such as foxes and felines cannot synthesize taurine endogenously and completely depend on the dietary sources of this amino acid. The taurine synthesis capability of the human liver is also negligible. The current study found that taurine level in various tissues and their isolated mitochondria was significantly depleted by cholestasis/cirrhosis. Taurine synthesis capability synthesis of the liver or its absorption through the intestinal brush border could be affected by cholestasis/cirrhosis. Lower taurine levels could lead to deleted amino acid stores in various organs and cause organ injury. These data could support the importance of strategies such as taurine supplementation in cholestatic/cirrhotic patients
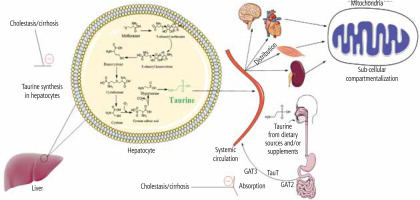
Another potential mechanism for decreased TAU levels of tissues and mitochondria in cholestasis/cirrhosis could be related to the changes in the expression and/or activity of TAU transporters (TauT). The cellular and mitochondrial uptake of TAU is mediated via specific TauT [83, 84]. Hence, monitoring the changes of these transporters during cholestasis/cirrhosis could give a better insight into the mechanisms of TAU depletion in various tissues (Fig. 4).
Cases of TAU deficiency have also been previously reported in association with different pathologies. It has been found that situations such as long-term paraenteral nutrition or acute exposure to cytotoxic agents (e.g., cancer chemotherapy regimens) could lead to TAU deficiency [223-225]. Today, the importance of TAU in regulating the physiological function of different systems is entirely approved by many experimental and clinical data. The current study found that tissue and mitochondrial TAU levels were significantly depleted during cholestasis/cirrhosis. Therefore, TAU supplementation could preserve various organs in a more functional state.
The safety of TAU and its application in critically ill patients (e.g., cirrhotic patients) is another subject that should be considered before the application of this amino acid in clinical settings. Fortunately, there are many studies on the application of TAU against various human diseases in clinic [226-228]. It has been found that TAU could be administered to humans at very high doses (e.g., 6-12 g/day) without any considerable side effect [228]. Interestingly, some studies demonstrated that TAU could be administered at high doses in disorders such as hepatic encephalopathy and cirrhotic patients (e.g., for controlling portal hypertension) [122]. These data indicate that TAU could be safely administered in clinical cases of cholestasis/cirrhosis. However, determining the long-term effects of TAU therapy in cholestatic/cirrhotic patients requires further studies.
The data obtained from the present research could provide an insight into the relevance of TAU deficiency in the pathogenesis of cholestasis/cirrhosis-induced organ damage and suggest a viable therapeutic option in patients. More studies, including clinical trials, could reveal the importance of TAU therapy in managing cholestasis/cirrhosis-induced complications.







