Introduction
Bronchial asthma (BA) is a chronic airway inflammation involving many cells and cellular components. This chronic inflammation leads to airway hyperresponsiveness, often exacerbated at night and early in the morning. Extensive and variable reversible airflow restriction is usually present, and most patients can relieve themselves or after treatment [1–3]. The clinical manifestations of BA patients are recurrent wheezing, shortness of breath, with or without chest tightness or cough, which are severe at night and after getting up. It is usually associated with exposure to allergens, cold air, physical or chemical stimulation, upper respiratory tract infection, exercise, etc. [4, 5]. Asthma is treated differently for different stages and grades, and the goal is to achieve both current control and future risk reduction. The treatment goals of acute attack are different from those of chronic duration. The goals of acute attack are to relieve symptoms as soon as possible, relieve airflow restriction, and improve hypoxaemia. The chronic duration therapy aims at good control of asthma symptoms, normal activity levels, and the lowest risk of transferring to an acute attack, impairment of lung function, and drug-induced events. BA causes relatively serious harm, disturbing many patients, causing abnormal breathing of patients, dizziness, fatigue, and other symptoms, and disturbing the normal life of patients. We should attach great importance to BA and clearly understand the symptoms of the disease [6–8].
BA is a disease with multi-gene genetic predisposition and is greatly influenced by environmental factors [9, 10]. Environmental factors include allergenic factors such as indoor, outdoor, occupational, food, drugs (aspirin and antibiotics), and non-allergenic factors [11–13]. Cytokines are small molecular polypeptides that regulates cell function. During immune response, cytokines are crucially involved in cell interaction, growth, and differentiation [14]. Interleukin (IL) is a cytokine that is produced by lymphocytes, monocytes, and other leukocytes rather than mononuclear cells and is critical in regulating cellular interactions, immune regulation, haematopoietic production, and inflammation. Tumour necrosis factor (TNF) was originally found to cause necrosis of tumour tissue and was named after it. Besides killing tumour cells, TNF also has immune regulation function and participates in the occurrence of fever and inflammation [15, 16]. Therefore, understanding and prevention of BA disease is the focus of clinical research.
In conclusion, the mechanism of action between serum cytokines and BA is the focus of clinical research. Therefore, this work selected 150 children for comparison and analysis with 3 groups. Fifty children suffered from BA in the acute attack stage, 50 children were with BA in remission stage, and another 50 children were healthy. The baseline data and levels of IL-33, IL-15, IL-4, and IFN-γ of the 3 groups were compared, and Spearman was used to detect the correlation of each serological index, which provided a new theoretical basis for the pathogenesis of BA in children.
Material and methods
Research objects
A total of 100 children with BA admitted to our hospital from 10 May 2021 to 10 August 2023 were included as research objects. The children were grouped into an acute attack group (AA group) and a clinical remission group (CR group) according to their medical history and clinical characteristics, with 50 children in each group. Then, 50 healthy children were randomly selected as controls. This clinical study was approved and implemented by the Ethics Committee of the hospital. All patients participated voluntarily and signed an informed consent form.
Inclusion criteria for children with BA were given as follows: no glucocorticoids in the last month; no immunomodulator taken within 1 month; the first wheezing episode; with other immune diseases; and combined history of allergic disease. Exclusion criteria were defined as follows: absence of clinical data; failure to cooperate with the interviewer; allergic to therapeutic drugs; and asthma associated with pulmonary tuberculosis or pulmonary tuberculosis.
Inclusion criteria for healthy children were as follows: under 2 years of age; no disease in the past month; no glucocorticoids taken within 1 month; and without antihistamines in the past month. Exclusion criteria were given as follows: under 2 years of age; abnormal mental state, unable to communicate normally; and missing baseline data.
Sample collection and preservation
4 ml of venous blood of AA group patients, CR group patients, and control patients were collected, left to stand at 25°C for 120 min, and then centrifuged at 2000 rpm for 15 min. Supernatant was retained and stored in the refrigerator at –70°C for follow-up test.
Detection methods
Levels of IL-33, IL-15, IL-4, and IFN-γ were detected by ELIA. First, the kit was kept at room temperature, the reaction plate was taken our, and standard 8 wells were set. The standard solution with different gradient concentrations was added to wells 1-7 successively. 100 μl sample dilution was added to the 8th well as a control. After the enzyme label plate was covered, it was placed at 37°C for 1 h. After the liquid in the plate was sucked, each well was added with 100 μl biotin anti-human antibody and then placed at 37°C for 1 h. After that, the reaction plate was rinsed, and then 100 μl of enzyme-labelled antibody was added again. Next, the reaction plate was cleaned at 37°C for 1 h. Again, the reaction plate was cleaned with washing solution 3 times. Then, after the 3,3 ‘,5,5 ‘-tetramethylbenzidine (TMB) colouring solution was added, the wells were cultured at 37°C for 30 min under the condition of no light. Then 100 μl TMB stopping solution was added so that the reaction could be stopped. After mixing, we measured the optical density (OD) at 450 nm using an enzyme labelling instrument. In addition, the concentration of the sample was calculated.
Statistical analysis
The data were processed with SPSS19.0. The measurement and count data were given in the form of mean ± standard deviation (`x ± s) and percentage (%), respectively. Also, we employed the t test and log-rank test to analyse the difference of baseline data among the 3 groups. Spearman was used to detect the correlation of each serological index. The difference was statistically significant with p < 0.05 in the bilateral test.
Results
Comparison of baseline data among 3 groups of children
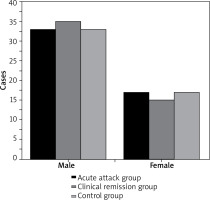
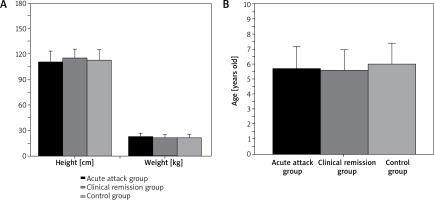
As shown in Figures 1 and 2 below, there were 50 children in the AA group, 33 boys and 17 girls, with mean age of 5.71 ±1.45 years, height of 110.66 ±12.15 cm, and weight of 22.75 ±3.82 kg. The CR group consisted of 50 patients, including 35 boys and 15 girls, with mean age of 5.58 ±1.37 years, height of 115.13 ±10.48 cm, and weight of 21.92 ±3.14 kg. In the controls, 50 children, including 33 males and 17 females, were 5.95 ±1.44 years old, 113.72 ±12.36 cm in height, and 21.35 ±2.43 kg in weight. In conclusion, pairwise comparison of male and female cases, age, height, and weight of AA group patients, CR group patients and control group children showed no great difference (p > 0.05).
Comparison of serum IL-4 levels in 3 groups of children
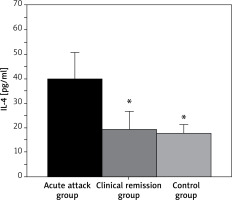
The IL-4 levels of children with BA in the AA group and CR groups were 39.52 ±11.13 pg/ml and 19.25 ±7.48 pg/ml, respectively, while that in controls was 17.68 ±3.55 pg/ml (Figure 3). In conclusion, the serum IL-4 level in the AA group was much higher and exhibited p < 0.05 to that of controls, but that in the CR group showed no statistical significance compared with that of controls (p > 0.05).
INF-γ levels in 3 groups of children
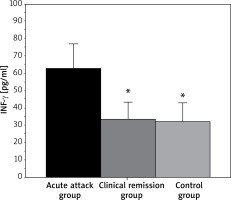
As shown in Figure 4, the serum INF-γ level in the acute attack patients was 62.77 ±14.05 pg/ml, and the serum INF-γ level in the clinical patients was 33.08 ±10.22 pg/ml. The INF-γ level in controls was 31.95 ±8.75 pg/ml. In conclusion, the INF-γ level in the AA group was higher and presented a difference with p < 0.05, while that in the CR group showed no statistical significance (p > 0.05) when they were compared to the level in children as controls.
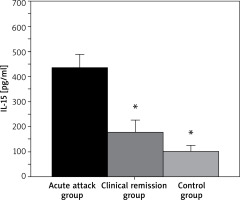
Serum IL-15 levels in 3 groups of children
As shown in Figure 5 below, IL-15 levels in the children with BA in the AA group and CR group were 435.18 ±50.33 pg/ml and 179.55 ±43.72 pg/ml, respectively, while that in the controls was 101.25 ±23.81 pg/ml. In conclusion, the children with BA in both the AA group and CR group exhibited an obviously higher IL-15 level with p < 0.05 in contrast the controls.
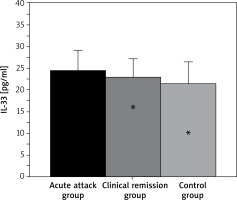
IL-33 levels among 3 groups of children
As shown in Figure 6 below, IL-33 levels in the acute attack patients and clinical remission patients were 24.55 ±4.63 pg/ml and 22.87 ±4.28 pg/ml, respectively, while in controls it was 21.49 ±5.01 pg/ml. In conclusion, IL-33 levels of the patients with AA group showed no obvious difference compared with those of children in the clinical remission stage of BA and healthy children (p > 0.05).
Correlation analysis of different serological indexes in children with BA
As shown in Table 1 below, the serum IL-4 level was positively linked with the INF-γ level (r = 15.621, p = 0.002) and with the IL-15 level (r = 9.581, p = 0.008), and the IL-33 level was not significantly correlated with the IL-33 level (r = 4.027, p = 0.074).
Table 1
Analysis of the relationship between serum IL-4 levels and INF-γ, IL-15, and IL-33 levels in children
| Indicator | INF-γ | IL-15 | IL-33 |
|---|---|---|---|
| r | 15.621 | 9.581 | 4.027 |
| P-value | 0.002 | 0.008 | 0.074 |
As shown in Table 2 below, we observed a positive and significant correlation between INF-γ level and IL-15 level (r = 17.316, p < 0.001), but no remarkable relationship was found between INF-γ level and IL-33 level (r = 2.811, p = 0.083).
Table 2
Analysis of the relationship between INF-γ level and IL-15 and IL-33 level in children
| Indicators | IL-15 | IL-33 |
|---|---|---|
| r | 17.316 | 2.811 |
| P-value | 0.000 | 0.083 |
Table 3 shows that the correlation between serum IL-15 level and IL-33 level was not obvious (r = 4.137, p = 0.067).
Discussion
At present, BA is becoming more and more common, this disease is extremely harmful, especially prevalent among children and adolescents, resulting in abnormal breathing of patients. Therefore, it is necessary to guard against the occurrence of BA and know the symptoms of the disease [17, 18]. Most patients will have an episodic cough, accompanied by chest tightness and dyspnoea symptoms. Patients with long-term disease are accompanied by severe symptoms of sputum production. During remission, the sputum production of patients increases significantly. If there is no co-infection, most of them have white phlegm, and some patients produce rice granular mucous [19]. The pathogenesis of BA has not been clearly defined in clinics, and the patient system, genetic factors, and environmental factors all have an impact on the development of the patient’s disease, so it is very important to understand the characteristics of children with BA [20–23]. According to the patient history and clinical characteristics, 100 children with BA were enrolled into an AA group and a CR group, with 50 children in each group. Then, another 50 healthy children were randomly selected as controls. Firstly, the baseline data of the children were compared, and it was found that pair-to-pair comparison of the number of cases, age, height, and weight of male and female patients of the AA group, CR group, and controls exhibited no obvious significance (p > 0.05). The balance of baseline data between groups is to ensure the comparability of observation results of response variables between groups, to investigate the real impact of treatment factors on observation results under similar baseline conditions, which provides feasibility for subsequent research results.
In this work, it was found that the IL-4 level of AA group children was much higher than that of children and control children (p < 0.05), but that of the CR group patients showed no obvious difference to controls (p > 0.05). Such result suggests that IL-4 factor level plays a pro-inflammatory role in BA [24, 25]. The serum INF-γ level of the patients in the AA group was the highest and exhibited a p < 0.05 difference with the other 2 groups with p > 0.05 between each other. This is similar to previous reports suggesting that INF-γ factor may play a pro-inflammatory role in childhood asthma through multiple pathways. IL-15 is a T cell growth factor produced primarily by monocytes and macrophages. IL-15mRNA is expressed in human heart, lung, and kidney, and especially placenta, muscle, and other tissues, which has a wide range of immunoregulatory activities and can participate in regulating the survival, proliferation, and function of a variety of immune cells [26]. It was found in this work that the IL-15 level of patients in the AA group was greatly elevated compared with children with BA in clinical remission stage and healthy children, while that between the latter 2 exhibited p < 0.05. These results indicate the importance of IL-15 in children with BA, which may be related to the persistence of chronic airway inflammation. In addition, the IL-33 levels of the patients in the AA group showed no statistical significance compared with clinical remission and control rates (p > 0.05). Such a finding is different from the study results of Li et al. (2021) [27], possibly due to the small sample size of children included in this work, leading to the inconsistency of the study results. Spearman analysis showed that the serum IL-4 level was positively linked with the INF-γ level (r = 15.621, p = 0.002) and the IL-15 level (r = 9.581, p = 0.008). This suggests that serum IL-4 factor is associated with IL-15 and INF-γ factor, and there is a synergistic relationship in BA promoting inflammation. In addition, the IL-33 level showed no obvious relationship with IL-4, IL-15, and INF-γ factors (p > 0.05). This is similar to the above results, suggesting that the role of serum IL-33 factor in children with BA needs to be further confirmed.
Conclusions
In this work, 100 children with BA treated in our hospital were included as research objects, and they were divided into an AA group and a CR group, with 50 children in each group. Fifty healthy children were selected as controls. The baseline data and levels of IL-33, IL-15, IL-4, and IFN-γ of the 3 groups were compared, and the correlation of each serological index was detected by Spearman. The results showed that serum IL-15, IL-4, and IFN-γ factors were associated with the onset of BA in children with acute attack and played a role in the exacerbation of respiratory tract inflammation. IL-4 factor is associated with IL-15 and INF-γ factor, and there is synergistic relationship in BA promoting inflammation. Unfortunately, the sample of children included in this work was small, and there were no more specific grading criteria, and there was no further analysis of the mechanism of serum IL-33, IL-15, IL-4, and IFN-γ factors on BA pathogenesis. Therefore, in future studies, we will re-include children with BA from several hospitals to explore the roles of cytokines in BA. In conclusion, this work provided reference for the clinical treatment of BA in children.