Introduction
The most common type of adult leukaemia is chronic lymphocytic leukaemia (CLL), which is characterised by abnormal growth and expansion of mature B-lymphocytes that express CD5/CD19/CD20/CD23-positive in the peripheral blood, bone marrow and lymphoid tissues [1-3]. Atypical neoplastic cells show various survival mechanisms implemented at the cellular level, e.g., avoidance of caspase-dependent apoptosis, B-cell receptor (BCR) signalling, and interaction between CLL cells and the microenvironment [4, 5]. The pathogenesis of CLL in most studies is not recognised; however, every case has a strong dependence on proliferation and the progressive accumulation of characteristic monoclonal B-lymphocytes. The disease is more common in men, and mainly affects the elderly. High genetic predisposition concerns, inter alia, a mutated type of variable heavy chain immunoglobulin (IGHV). However, no common single-gene mutation is directly related to the detection of the CLL [3]. The detection of some cytogenetic and molecular anomalies is of significant prognostic importance, which results in longer or shorter survival of the patient than would result solely from the classic clinical progression [4] according to the Rai [6] or Binet classification [7]. The mutation with the worst prognosis for patients is the presence of del17p or a mutation in the TP53 gene. Breakthrough drugs in the treatment of chronic lymphocytic leukaemia can be divided into three groups with different mechanisms of action: anti-CD20 monoclonal antibodies (rituximab, obinutuzumab and ofatumumab), BCR inhibitors (ibrutinib and idelalisib) and inhibitors of Bcl-2 protein (venetoclax). Unfortunately, CLL is still not a curable disease, despite advances in therapy and improved treatment outcomes [3, 4].
Immune suppression promoted both by therapies used and high-risk disease progression leads to many complications including autoimmune complications, infection- related immunodeficiency, secondary neoplasms, and Richter transformation (RT) [3]. Richter’s syndrome occurs in 2.8% to 10.7% of all CLL patients, associated with the disease. It is described as high-grade lymphoma, with poor prognosis and refractory to treatment. The normally slow growing, or indolent, CLL converts to diffuse large B-cell lymphoma (DLBCL), but it could also progress to Hodgkin’s disease, prolymphocytic leukaemia, or acute lymphoblastic leukaemia (ALL), and some types of T-cell lymphomas have also been reported [8, 9]. Richter transformation is characterised by a sudden, progressive lymphadenopathy and rapid onset of symptoms such as weight loss, night sweats, and fever. Moreover, a rapid increase in serum LDH concentration, advanced Rai stage, ZAP-70 expression, IGHV1 unmutated disease, hypercalcemia, high serum β2 microglobulin (β2M) level or del(17p) levels may herald the development of Richter transformation [9, 10]. However, these clinical symptoms are observed only in 50-60% of RT cases. The basis of the diagnosis is histological documentation with a biopsy in the presence of a large nodal mass or positron emission tomography (PET) [11, 12].
Case report
A 62-year-old man with signs of progression of the underlying disease (chronic lymphocytic leukaemia, CLL) was admitted to the Department of Haemato-oncology and Bone Marrow Transplantation in Poland in February 2010. According to the patient’s medical history, the neoplasm was diagnosed in 2006 in Rotterdam, the Netherlands. Until 2010, the patient was treated with chlorambucil in a foreign clinic. Necessary tests were performed, including the determination of the immunophenotype. Laboratory tests showed a slightly increased WBC level (11.87 K/µl), LUC level (0.6 K/µl) and number of lymphocytes (LYM) (5.36 K/µl) (45.1% CD5+23+19+ B-cells), while the LDH level (280 IU/l) and β2M level (1.88 mg/l) were in the normal range, with a normal count of red blood cells and platelets. The diagnosis of CLL according to Rai’s stage 2 was maintained in accordance with the initial diagnosis and the FC cytostatic system (cyclophosphamide + fludarabine) was used in ambulatory treatment. From March 2013, the patient was treated with human normal immunoglobulins in subcutaneous administration due to the reduced levels of IgM (4.85 g/l), IgM (0.14 g/l), and IgA (0.34 g/l). In 2015, the patient was urgently admitted to the ward with suspicion of disease progression – weakness, sweating, peripheral lymphadenopathy, hepatosplenomegaly in the ultrasound (USG). Laboratory tests revealed massive leucocytosis (WBC – 68.5 K/µl). Virological tests for HCV and HBV were negative. Immunophenotype and cytogenetic tests were ordered. The flow cytometry of peripheral blood confirmed the domination of monoclonal B-cells – 80% lymphocytes with the presence of antigens: CD5+, CD19+, CD23+, CD20+, CD38+/–, CD200+. Genetic testing showed absence of characteristic mutations. Associated diseases: paroxysmal atrial fibrillation, type 2 diabetes, erosive gastritis (Helicobacter pylori positive), atherosclerosis, etc. The patient was qualified for immunochemotherapy in the BR scheme (rituximab + bendamustine), and from the second half of 2016, doxorubicin, vincristine, cyclophosphamide, prednisone in the CHOP scheme. Partial remission of the disease was achieved. However, in the second half of 2017, due to the increase in WBC to 131 K/µl, it was decided to administer rituximab and ibrutinib. The presence of anti-HBc total antibodies was detected and lamivudine was introduced prophylactically. The leukogram (Fig. 1 [13]) and peripheral blood immunophenotype results were presented by 43% of CD5+, CD19+, CD23+, CD20+, CD38+/–, CD200+, CD22+, CD43+, CD71–, sIgM–, sLambda–, sKappa+, ZAP-70± B-cells (Fig. 2). Due to the diagnosed atelectatic-inflammatory changes in the middle and lower fields of the right lung in computed tomography (CT), the patient was referred for bronchoscopy, which was performed in 2017. After obtaining preliminary results of the bronchoscopy, Candida tropicalis was detected. It was decided to use a fluconazole antifungal treatment. The final histopathological diagnosis of mediastinal nodes collected during the bronchoscopy also revealed infiltrates corresponding to chronic lymphocytic leukaemia. In a 2018 ultrasound of the abdominal cavity, the liver was still expanded, with enlarged lymph nodes along the vessels and in the hilum of the liver (1.09 × 0.77 cm and 1.14 × 0.53 cm). The patient remained under constant care of the Haemato-oncology Clinic.
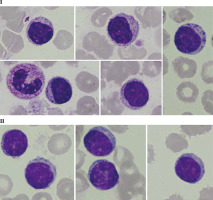
Fig. 1
Peripheral blood smear with monoclonal B-cells and smudge cells (Gumprecht shadows [13]) characteristic of CLL. Photos were obtained from the resources of the Department of Haemato-oncology and Bone Marrow Transplantation, Poland

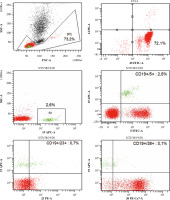
Fig. 2
Analysis of the patient’s peripheral blood immunophenotype from 2017. The diagnostic panel for B-NHL disorders contained anti-human mouse monoclonal antibodies CD45-FITC, CD14-PE, for the present patient. Tube II was composed of a combination of CD5-FITC, CD23-PE, CD38-Per-Cy7, CD19-APC, CD20-APC-H7. Tube III was composed of a combination of CD22-FITC, CD43-PE, CD19-Per-Cy7, CD25-APC. Tube IV was composed of a combination of CD200-FITC, sIgM-PE, CD19-Per-Cy7, CD25-APC. Tube V was composed of a combination of sLambda-FITC, sKappa-PE, CD19-Per-Cy7 extracellularly marked (BD Biosciences, US). An acquisition gate was put on lymphocytes in keeping with forward and side scatter (respectively: FSC, SSC) qualities. Lymphocytes accounted for 64.8% of peripheral blood cells. The positive subpopulation CD19+ was chosen from gate P2 (43.6%) and was set up individually with each of the markers: CD5+ (43.3%), CD23+ (31.8%), CD38+ (21.0%), CD20+ (42.2%), CD10– (0.1%), CD43+ (45.8%), CD200+ (43.7%), sIgM– (0.7%). Selective gating of this lymphocyte population reveals them to be monoclonal for surface Kappa Ig light chains (31.7%) and that they lack expression of surface Lambda Ig light chains. Nevertheless, the obtained immunophenotype profile is the basis for the recognition of monoclonal B-lymphocytes which are characteristic of chronic lymphocytic leukaemia. **B-NHL – B-cell non-Hodgkin lymphoma; s – surface, Ig – immunoglobulin, FITC – fluorescein isothiocyanate, PE – phycoerythrin, Per-Cy7 – peridinin cyanin7, APC – allophycocyanin, H7 – Hillite7


Due to the described inflammatory changes in the ultra- sound of the colon, a colonoscopy was recommended during the stay in the gastroenterology ward. In the collected specimen sent for histopathological examination, there was found, inter alia, an Epstein-Barr virus (EBV)- positive mucocutaneous ulcer with accompanying lesions of inflammatory bowel disease. In 2021, the patient stayed at the Department of Clinical Immunology due to deep hypogammaglobulinaemia (IgG – 1.83%) in the course of the underlying disease, in connection with recurrent infections requiring frequent antibiotic therapy. The performed tests revealed unregulated diabetes (glycated haemoglobin HbA1c – 10.9%), slight leucocytosis with lymphocytosis (WBC – 11.68 K/µl, LYM – 7.27 K/µl (62.2%)), microcytic anaemia (RBC – 5.06 G/µl, HGB – 10.7 g/dl, HCT – 35.8%, MCV – 70.8 fl), transferrin and iron deficiency (Tf – 1.5 g/l [N: 2.0-3.6], Fe – 7.4 µg/ml [N: 50-212]), increased inflammatory parameters (calprotectin – 473.1 µg/g [N: < 120], CRP – 44.0 mg/l). Moreover, active CMV (CMV-DNA – 6.02 × 102 CMV copies/ml) and EBV (EBV-DNA – 3.12 × 104 DNA copies/ml) infection was found and valaciclovir was included in the treatment. Based on the viraemia result, sequential treatment of rituximab was started once a week. Control viral load testing after 2 doses of rituximab showed a reduction of viral load to 2.82 × 103 DNA copies/ml. A history of significant weight loss, weakness, low-grade fever and abdominal pain was revealed. The patient underwent specialist diagnostics again due to the suspicion of disease progression. Cells with the immunophenotype characteristic of CLL were excluded. An analysis of marker expression using the flow cytometry of peripheral blood showed the presence of approximately 48.2% natural killer cells (NK cells) with CD2+, CD7+, CD3–, CD16+, CD56+, CD8+/–, CD4–, Cyt3+/–, CD38+, CD43+, CD5–, CD19–, CD30–, CD34–, TdT– immunophenotype (Fig. 3). Bone marrow trepanobiopsy was performed, and imaging examinations were updated. The presence of about 10.1% NK cells with the immunophenotype as above was confirmed in the bone marrow, and in the myelogram lymphocytes that may correspond to the morphology of NK cells were observed (Fig. 4). The trepanobiopsy results confirmed the infiltration of small lymphoid cells (CD45+), which constitute about 20% of the bone marrow cells, about 1/3 of which are T-cells (CD3+, CD5+, CD8+, CD4–, CD20–, CD23–). There were no infiltrations characteristic of CLL. Due to the lack of access and the insufficient size of the lymph nodes, it was impossible to collect material for histopathological examination after a surgical consultation. Currently, based on the overall clinical picture, the progression of chronic lymphocytic leukaemia has been ruled out. Lymphoproliferation of NK cells with bone marrow involvement was diagnosed. Treatment of basic diseases was continued, as well as antiviral and antitumor treatment (ibrutinib, rituximab, Dexaven). Unfortunately, despite the treatment, the patient died due to a critical general condition.
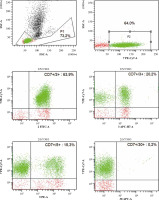
Fig. 3
Re-analysis in 2021 of the patient’s peripheral blood immunophenotype due to suspicion of disease progression. The diagnostic panel for B-NHL disorders contained anti-human mouse monoclonal antibodies CD45-FITC, CD14-PE, for the present patient. Tube II was composed of a combination of anti-CD5-FITC, anti-CD23-PE, anti-CD38-Per-Cy7, anti-CD19-APC, anti-CD20-APC-H7. The diagnostic panel for T-lymphocyte and NK-lymphocyte disorders contained anti-CD2-FITC, anti-CD5-PE, anti-CD7-Per-Cy7, anti-CD30-APC, anti-CD3-APC-H7 (Tube III). Tube IV was composed of a combination of anti-CytTdT-FITC, anti-CD2-PE, anti-CD7-Per-Cy7, anti-Cyt3-APC. Tube V was composed of a combination of anti-CD3-FITC, anti-CD8-PE, anti-CD45-Per-Cy7, anti-CD4-APC. Tube VI was composed of a combination of anti-3-FITC, anti-CD16+56-PE (BD Biosciences, US). An acquisition gate was put on lymphocytes in keeping with forward and side scatter (respectively: FSC, SSC) qualities. Lymphocytes accounted for 72.1% of peripheral blood cells. The positive subpopulation CD19+ was chosen from gate P2 (2.6%) and was set up individually with each of the markers: CD5+ (2.6%), CD23+ (0.7%), CD38+ (0.1%). B-cell lymphoproliferation was excluded. The diagnostic panel towards T/NK-lymphocyte disorders has been extended. The positive subpopulation CD7+ was chosen from gate P2 (64.0%) and was set up individually with each of the markers CD2+ (63.9%), CD3+ (20.2%), CD5+ (15.3%), CD30– (0.2%), Cyt3+/– (34.1%), CytTdT– (0.0%). In the last stage of the analysis, the CD56+16+ cells were gated (49.4%), which showed lack of CD3 (1.2%), CD4 (0.0%) and partially CD8 (17.3%) expression. Marker expression analysis using the flow cytometry of peripheral blood showed the presence of approx. 48.2% NK cells with CD7+, CD2+, CD3–, CD5–, CD30–, Cyt3+/–, TdT–, CD8+/–, CD4–, CD16+, CD56+ immunophenotype. ** Cyt – cytoplasmic

Discussion
In the vast majority of cases chronic lymphocytic leukaemia is an incurable disease. The aim of therapy is to control disease progression, prolong life and improve its quality, similarly to other indolent neoplasms of the lymphatic system [14]. Recurrent infections are very frequently observed and are the most common cause of death in patients. The causes are immune disorders related not only to leukaemia, but also to anti-cancer treatment, concomitant diseases (e.g., diabetes) and age. Immunity disorders involve, among other things, the inhibition of B lymphocyte proliferation and reduced production of antibodies (hypogammaglobulinemia) (the humoral response), but also lead to abnormal amounts and dysfunction of T and NK cells (the cellular response). Moreover, disturbances in phagocytic, chemotactic and bactericidal functions as well as a reduced number of neutrophils and various enzymes [15, 16] lead not only to increased susceptibility to infections and a weakened response to vaccinations, but also to the occurrence of secondary neoplasms and the development of autoimmune complications. The spectrum of infections depends on the clinical stage of the disease. In patients who are in the first stage of the disease, bacterial and fungal infections predominate. Herpes virus infections are reactivated relatively often. Anti-CD20 monoclonal antibodies increase the risk of reactivation of hepatitis B virus and JC (John Cunningham) virus. Further treatment influences the reactivation of CMV and EBV infection [16].
It is estimated that around 85,000 lymphomas are caused by infections worldwide each year [17]. The main infectious factors associated with the etiopathogenesis of these diseases include, among others, lymphotropic viruses, including the Epstein-Barr virus and factors causing chronic stimulation of the immune system including hepatitis B virus (HBV) or H. pylori [18]. Probably NK-cell lymphoproliferative disease, in the described case, was induced by the patient’s immunodeficiency correlated with active EBV infection or as a result of CLL transformation into another more aggressive lymphoma. The patient diagnosed with chronic lymphocytic leukaemia was treated with multiple regimens in long-term immunosuppression with CMV, EBV and H. pylori re-infection. In immunocompetent patients, the smooth functioning of the cellular response regulated by T cells prevents uncontrolled proliferation of infected B cells and EBV replication. In contrast, patients with immunodeficiency or those undergoing immunosuppression are at increased risk of developing lymphoma due to the lack of immune surveillance over the proliferation of EBV-infected B lymphocytes [19]. As previously mentioned, EBV infection also directly affects NK cells. The most common EBV+ NK-cell malignancy is NK-cell lymphoma, nasal type, which usually presents extranodally in the upper aerodigestive tract. However, there are less common EBV+ leukaemias with different clinical presentations and biology [20, 21]. According to Tse and Kwong, NK-cell lymphoma occurs most often (80%) as a nasal type, less frequently (20%) in non-nasal areas (salivary gland, gastrointestinal tract, skin), and hardly ever as a disseminated disease with a leukaemic phase [22]. Suzuki et al. observed a relationship between EBV infection and aggressive NK-cell lymphoma. Over 90% of cases show clonal, episomal (contained in the cytoplasm) forms of EBV. Most cases are diagnosed in young adults with involvement of peripheral blood, bone marrow, liver and spleen. The average survival time of patients is often less than 2 months [23]. In addition, the abnormal proliferation of mature NK cells, which is almost always EBV related, has been classified by the WHO as aggressive natural killer-cell leukaemia (ANKL) with a median overall survival of seven months. Generally, the Epstein-Barr virus (EBV)-associated NK-cell lymphoproliferative disease has been linked to poor prognosis and a treatment strategy has yet to be established [24].