Introduction
Cervical carcinoma represents the fourth most common malignancy and the fourth leading cause of cancer deaths in the world [1]. In developing countries, cervical cancer is the second cause of cancer-associated death in females, particularly those under 20–39 years old [2, 3]. Deaths in these patients are usually due to invasion and metastasis due to the aggressive nature of the tumour [4]. Early diagnosis of cervical carcinoma can enhance the effectiveness of treatment and show better outcome, with a 5-year survival rate of 90% [5]. Therefore, it is critical to identify more potent therapeutic targets and new prognostic markers to detect high-risk patients early and treat them early.
Protein disulphide isomerase A3 (PDIA3) is a chaperone protein that is localised to the endoplasmic reticulum (ER). Under normal conditions, PDIA3 forms a complex with calreticulin and calnexin to correct folding of misfolded proteins in ER and new synthetic glycoproteins. This protein inhabits ER stress-induced apoptosis [6]. Furthermore, it is involved in stabilisation of MCH-I, modulating mechanistic target of rapamycin (mTOR) complex and regulation of signal transducer and activator of transcription 3 (STAT3) transcriptional potential [7]. Protein disulphide isomerase A3 is suggested to be upregulated in various cancers, such as hepatocellular carcinoma [8], gastric cancer [9], and glioma [10]. Its expression is correlated with the outcome of malignancy.
The signal transducer and activator of transcription 3 is the major pathway implicated in development and progression in HPV-positive cervical carcinoma. This pathway is triggered by inflammatory cytokines and growth factors [11, 12]. Tyrosine phosphorylation activates STAT3, resulting in the formation of a dimer (p-STAT3) that moves to the nucleus to bind DNA and promote the expression of genes that are essential in the proliferation, differentiation, and apoptosis of cells. It also controls cell response to hypoxia by angiogenesis in various cancers [13–15].
Protein disulphide isomerase A3 binds with STAT3 in the nucleus. Protein disulphide isomerase A3-STAT3 complex is positively correlated with tumour progression in several tumours and is associated with radiotherapy resistance in laryngeal carcinoma by modification of STAT3 activity [16]. The aim of the work was to assess immunohistochemistry expression of PDIA3 and p-STAT3 in cervical carcinoma, and to investigate their correlation with the clinicopathological characteristics.
Material and methods
The present prospective cohort study conducted on 50 female patients of cervical cancer, where they operated at the General Surgery and Gynaecology & Obstetrics departments of Zagazig university hospitals by total hysterectomy with bilateral salpingo-oophorectomy, either with or without pelvic lymph-adenectomy, from January 2019 to January 2023. No cases received pre-operative anticancer therapy. The clinical data were gathered from pathology reports, including age, presentation, and imaging investigations as computed tomography of the pelvic abdomen and chest, and a bone scan. At the pathology department, tumour grade and stage were evaluated according to the International Federation of Gynaecology and Obstetrics (FIGO) grading and staging system and the eighth edition (2018) of the American Joint Committee on Cancer [17].
We evaluated the patient’s survival corresponding to follow-up data from the clinical records of medical and clinical oncology departments. Overall survival (OS) was assessed as time from surgical intervention to the death or the last follow-up. Disease-free survival (DFS) was assessed from end of treatment to the tumour relapse or most recent patient follow-up (censored). The ethical approval was attained from the local institutional board of Zagazig University hospitals (ethics code #11266).
The adjuvant treatment
The type of adjuvant treatment used depends on the tumour stage and surgical findings. For patients with tumour stage IA, IB, or IIA1 with free surgical margins, negative lymph nodes (LN), negative parametrial involvement, and in whom there were no cervical risk aspects after radical hysterectomy (the Sedlis criteria), observation was preferred. On the other hand, radiation therapy with (or without) concurrent platinum encompassing chemotherapy was delivered if there were positive nodes, positive margins, positive parametria, large primary tumour, deep stromal invasion, and/or lymph-vascular space invasion. Definitive chemo-radiotherapy was delivered for medically inoperable cases or those who refused surgery.
Stage IIB and stage III were treated with surgery plus adjuvant radiotherapy with (or without) concurrent chemo- radiotherapy or definitive concurrent chemoradiotherapy. Stage IVA was treated with definitive concurrent chemoradiotherapy. External beam radiotherapy was delivered by 3-dimensional conformal technique using a total dose 40–50 Gy to the pelvis with a daily fraction size of 2 Gy 5 times/week. External beam radiation therapy (EBRT) was administered through a 4-field technique utilising a linear accelerator with a multi-leaf collimator and a conventional fractionation delivery in 20–25 days. The treatment fields either included the pelvic lymphatics or pelvic and paraaortic lymphatics in patients with para-aortic nodal metastasis. The gross involved LN were boosted with 10–20 Gy of high conformal EBRT. In definitive radiation therapy, the primary cervical tumour was boosted using brachytherapy with an additional 30–40 Gy. Concurrent chemotherapy administrated weekly comprised cisplatin in the dose of 40 mg/m2 or weekly carboplatin AUC 2 for patients who were cisplatin intolerant.
Immunohistochemistry
The Dako EnVision™ kit of polymer envision detection system (Dako, Copenhagen, Denmark) was used. 3–5-µm tissue sections of formalin-fixed paraffin-embedded tissue blocks were deparaffinised, then rehydrated, and lastly incubated for 10 min in antigen retrieval solution (pH 6.0). Finally, the slides incubated with monoclonal PDIA3 antibody (OTI4D7 Invitrogen, Waltham, 1 : 150) and monoclonal p-STAT3 antibody (D3A7 Cell Signalling Technology, Danvers, MA, USA 1 : 100). The response was envisioned by incubating the tissue slides with DAB 15 min, and then after Mayer’s haematoxylin was used.
The cytoplasmic PDIA3 expression was scored according to the percentage of positive cells, into 0 for ≤ 5%, 1: 6–30%, 2: 31–80%, and 3: > 80%. The intensity scored 1–3 and the final score ranged from 0 to 9, considered as low (0–2) or high expression (3–9) [18].
Nuclear p-STAT3 immunoexpression was categorised according to the percentage of nuclear stained cells stained as follows: 0–50% positive tumour cells as negative, and > 50% positive tumour cells as positive [19].
The statistics
The studied continuous parameters were expressed as mean ±SD and median, but the studied categorical variables were expressed as percentages that compared by Fisher’s exact test or Pearson’s χ2 test. The stratification of DFS and OS was organised according to the studied immunohistochemical biomarkers. The time-to-event distributions were valued by Kaplan-Meier plot and compared via 2-sided exact log-rank test. All the used tests were 2 sided. All our statistics were finished using SPSS 22.0 for Windows and MedCalc for Windows.
Results
Patients’ characteristics
The mean age of patients was 52.24 ±61.14 years (range 40–65). Among the cases, 74% were squamous cell carcinoma while 26% were adenocarcinoma. FIGO grade III was predominant among the cases (36%). Parametrial invasion, nodal metastasis, and lymph-vascular invasion were noted in 72%, 64%, and 80 of cases, respectively. The majority of the patients (54%) presented at advanced stages (FIGO III–IV). The median duration of follow-up was 28 months (range 11–36 months), during which 15 cases (30%) died and 11 patients had a recurrence of tumour. A summary of clinicopathological features of involved cases is shown in Table 1.
Table 1
Clinicopathological features, immunohistochemical markers, and outcome of 50 patients with cervical carcinoma
[i] CCRT – concurrent chemoradiotherapy, FIGO – International Federation of Gynaecology and Obstetrics, LN –lymph node, LVSI – lymph-vascular space invasion, p-STAT3 – phosphorylated signal transducer and activator of transcription 3, PDIA3 – protein disulphide isomerase A3 Categorical variables were expressed as number (%). Continuous variables were expressed as mean ±SD and median (range).
Association between protein disulphide isomerase A3 and phosphorylated signal transducer and activator of transcription 3 immunoexpression and clinicopathological features
Studying PDIA3 staining in the studied cases shows that there were 25 (50%) cases with PDIA3 high expression and 25 (50%) cases with PDIA3 low expression. The relationship between PDAI3 expression and different clinicopathological parameters revealed positive correlation between high PDAI3 expression and tumour grade (p < 0.001), and large tumours (≥ 4 cm) tended to show high expression more than small tumours < 4 cm (p = 0.010) (Fig. 1).
Fig. 1
Immunohistochemical expression of protein disulphide isomerase A3 in cervical carcinoma: low cytoplasmic expression in squamous cell carcinoma of the cervix (grade I) (400×) (A), high cytoplasmic expression in squamous cell carcinoma of the cervix (grade II, 400×) (B), high cytoplasmic expression in squamous cell carcinoma of the cervix (grade III, 400×) (C)
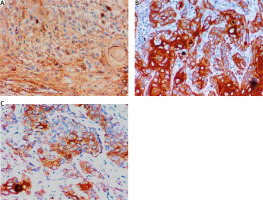
As regards histological types, 76.9% of adenocarcinoma cases and 59.5% of squamous cell carcinoma showed high PDIA3 expression with statistically significant association (p = 0.024). Furthermore, high PDIA3 expression was noticed to be related to depth of cervical stromal invasion (p = 0.017), lymph-vascular invasion (p = 0.005), parametrial invasion (p < 0.001), LN metastasis (p < 0.001), and higher FIGO stages (p < 0.001).
On the other hand, positive nuclear expression of p-STAT3 was detected in 22/50 (44%) of cases showing significant association with histological grade (p = 0.036), tumour stage (p = 0.021), LN metastasis (p = 0.020), and parametrial invasion (p = 0.045) (Fig. 2, Table 2, 3).
Fig. 2
Immunohistochemical expression of phosphorylated signal transducer and activator of transcription 3 in cervical carcinoma: nuclear expression in well differentiated squamous cell carcinoma of the cervix (400×) (A), nuclear expression in moderately differentiated squamous cell carcinoma of the cervix (400×) (B), nuclear expression in adenocarcinoma of the cervix (grade I, 400×) (C), nuclear expression in adenocarcinoma of the cervix (grade II, 400×) (D)
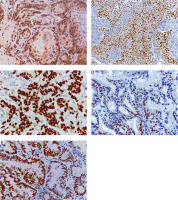
Table 2
Relation between clinicopathological features and immunohistochemical staining for phosphorylated signal transducer and activator of transcription 3 nuclear and protein disulphide isomerase A3 in cervical carcinoma patients
[i] FIGO – International Federation of Gynaecology and Obstetrics, LN – lymph node, LVSI – lymph-vascular space invasion, p-STAT3 –phosphorylated signal transducer and activator of transcription 3, PDIA3 – protein disulphide isomerase A3 Continuous variables were expressed as the mean ±SD and median (range). Categorical variables were expressed as number (%).a χ2 test b Mann-Whitney U test c χ2 test for trend p-value < 0.05 is significant
Table 3
Relation between clinicopathological features and immunohistochemical staining for phosphorylated signal transducer and activator of transcription 3 nuclear/protein disulphide isomerase A3 co-expression in cervical carcinoma patients
[i] FIGO – International Federation of Gynaecology and Obstetrics, LN – lymph node, LVSI – lymph-vascular space invasion, p-STAT3 – phosphorylated signal transducer and activator of transcription 3, PDIA3 – protein disulphide isomerase A3 Continuous variables were expressed as the mean ±SD and median (range). Categorical variables were expressed as number (%). a 2 test bMann-Whitney U test χ2 test for trend p-value < 0.05 is significant
Treatment outcome, survival, and progression analysis
High expression of PDIA3 was positively correlated with poor response to concurrent chemoradiotherapy with a progressive disease, while cases showing low PDIA3 expression achieved a complete response in 90.9% of cases (p = 0.001). Moreover, patients with negative p-STAT3 expression showed better treatment response than cases with positive p-STAT3 expression. Likewise, it was found that the higher PDIA3 expression was significantly associated with tumour relapse, poor DFS, and poor OS. Significant association was observed between p-STAT3 positivity and relapse (p = 0.002), mortality (p = 0.035), DFS (p < 0.001), and OS (p = 0.02).
Statistical analysis of the patient’s survival data revealed that shorter OS and DFS were associated with high PDIA3 expression and positive p-STAT3 immunoexpression (Table 4, 5). Kaplan-Meier survival curves that were utilised for PDIA3 expression and p-STAT3 immunoexpression are presented in Figures 3 and 4.
Table 4
Relation between immunohistochemical staining for phosphorylated signal transducer and activator of transcription 3 nuclear and protein disulphide isomerase A3 and outcome in cervical carcinoma patients
[i] CCRT – concurrent chemoradiotherapy, DFS – disease-free survival, OS – overall survival, p-STAT3 – phosphorylated signal transducer and activator of transcription 3, PDIA3 – protein disulphide isomerase A3 Continuous variables were expressed as mean (95% CI). Categorical variables were expressed as number (%).a χ2 test b Log rank test p < 0.05 is significant
Table 5
Relation between immunohistochemical staining for phosphorylated signal transducer and activator of transcription 3 nuclear/protein disulfide isomerase A3 and outcome in cervical carcinoma patients
[i] CCRT – concurrent chemoradiotherapy, DFS – disease free survival, OS – overall survival, p-STAT3 – phosphorylated signal transducer and activator of transcription 3, PDIA3 – protein disulfide isomerase A3 Continuous variables were expressed as mean (95% CI). Categorical variables were expressed as number (%). a χ2 test b Log rank test p < 0.05 is significant
Fig. 3
Kaplan-Meier curves of disease-free survival stratified according to protein disulphide isomerase A3 (PDIA3) immunohistochemistry (IHC) expression (A), phosphorylated signal transducer and activator of transcription 3 (p-STAT3) IHC expression (B), and combined expression of PDIA3 and p-STAT3 (C)
p-STAT3 – phosphorylated signal transducer and activator of transcription 3, PDIA3 – protein disulphide isomerase A3

Fig. 4
Kaplan-Meier curves of overall survival stratified according to protein disulphide isomerase A3 (PDIA3) immunohistochemistry (IHC) expression (A), phosphorylated signal transducer and activator of transcription 3 (p-STAT3) IHC expression (B), and combined expression of PDIA3 and p-STAT3 (C)
p-STAT3 – phosphorylated signal transducer and activator of transcription 3, PDIA3 – protein disulphide isomerase A3

A significant association between p-STAT3 and higher PDIA3 expression was noted (p = 0.001). Combined expression of PDIA3 and p-STAT3 was significantly associated with worse malignant criteria as high tumour grade (p < 0.001), LN metastasis (p < 0.001), vascular invasion (p = 0.026), and advanced stage (p < 0.001) in cervical carcinomas with worse OS (p < 0.001).
Discussion
Cervical cancer is a complex disease closely related to high-risk HPV infection; despite the great advances in therapeutic strategies its prognosis has not improved, and the mortality rate is still high [20, 21]. The survival rate of localised cervical carcinomas (stage IB1) is 91.6% [22]. Thus, it is important to find prognostic markers and new targets for therapy to improve the long-term prognosis of cervical carcinoma.
Protein disulphide isomerase A3, also known as ERp57/GRP58, is oxidoreductase enzyme that promotes oxidative folding of glycoprotein. Protein disulphide isomerase A3 is critical in major histocompatibility complex (MHC) class I, calcium homeostasis, apoptotic signalling, mTOR complexes, and promoting monocyte/macrophage differentiation [23]. The current study revealed that high PDIA3 expression was noted in 50% of the cases with higher expression comparable to normal tissue, suggesting that higher PDIA3 expression may play an essential role in the development of cervical carcinoma.
Additionally, we found that PDIA3 overexpression was significantly correlated with poor clinicopathological characteristics including tumour size, high tumour grade, deep stromal invasion, lymph-vascular invasion, parametrial invasion, LN metastasis, and advanced FIGO stage. Furthermore, patients with high PDIA3 expression showed significantly lower DFS and OS rates than patients with low expression. Our results are in line with Zhang et al. [18], who found that PDIA3 was highly expressed in 60.4% and was significantly higher than that in normal tissue (p < 0.05) and showing strong correlation with pathological type, progression of tumour stage, lower OS, and DFS, suggesting that high expression of PDIA3 can be used as an indicator of poor prognosis of cervical carcinoma.
Similarly, Liao et al. [24] found that expression of PDIA3 increased in 73% of cervical carcinomas. It was intense in adenocarcinoma compared with squamous cell carcinoma (p < 0.05), depth of cervical stroma invasion, and lower OS (p = 0.007 and RFS (p = 0.013), as confirmed by Rong et al. [25]. These findings are consistent with previous reports suggesting that higher PDIA3 expression had poor survival prognosis in diffuse glioma [10], renal clear cell carcinoma [26], and non-small cell lung cancer [27]. Takata et al. [28] suggested that PDIA3 might be a key molecule in new targeted therapies for hepatocellular carcinoma.
On the other hand, this finding disagrees with previous reports on early-stage gastric cancer [9], endometrial carcinoma [29], and papillary thyroid carcinoma [30] revealing that high PDIA3 expression was correlated with favourable prognosis. This might be due to dysfunction of the MHC class I complex. Protein disulphide isomerase A3 forms a complex with MHC class I to activate an immune response against the tumour. In contrast, when MHC class I expression is down-regulated, tumour cells avoid the cytotoxic effects of immune cells [9, 31].
Constitutive activation of STAT3 (p-STAT3) promotes cancer by directly regulating oncogene expression, such as cyclin B1, CDC2, p53, MCL-1, Survivin, VEGF, BCL2, and BAX. In addition, activated STAT3 down-regulates the expression of mediators critical for activation of the immune system against cancer. The key roles of tumour promotion and immunosuppression in the STAT3 pathway make STAT3 an important target for effective immunotherapy [32].
In this study, positive p-STAT3 was expressed in 44% of cases of cervical carcinoma and was significantly associated with high grade, LN metastases, and advanced FIGO stage. Shukla et al. [33] found that 56% of cervical carcinoma were positive for p-STAT3. Some studies reported different expressions of p-STAT3 in cervical carcinoma: Takemoto et al. [34] showed that 56% were positive for p-STAT3, while Choi et al. and Wu et al. [19, 35] reported that positive nuclear p-STAT3 was 67% and 78.3%, respectively. Chen et al. [36] reported 24%, which may be explained by their use of preserved tissue blocks or tissue arrays or different methods for assessing pSTAT3.
Our findings are in line with those of reported studies that have shown that positive expression of nuclear p-STAT3 was correlated with bad prognosis and decreased survival, as it was closely linked to high grade, presence of LN metastases, invasion, advanced stage, reduced OS, low disease-specific survival, and low relapse-free survival by functioning as a tumour promoter. Based on these findings, p- STAT3 might be a potential marker for poor prognosis and metastasis, and a therapeutic target for cervical cancer [19, 33, 35, 37].
We found a positive correlation between PDIA3 and p-STAT3 expression, which was significantly associated with worse malignant criteria such as high tumour grade, LN metastasis, vascular invasion and advanced stage in cervical carcinoma, and worse OS. Protein disulphide isomerase A3, unlike other PDI family members that are only localised in ER, was also found in other subcellular locations where PDIA3 forms a complex with p-STAT3 and facilitates transportation STAT3 to the nucleus. In addition, PDIA3 binds to DNA and enhances DNA-binding of STAT3 complex to promote transcription oncogenic genes [38]. Knockdown of those markers might improve the management of cervical carcinoma and inhibit tumour invasion, metastases, and recurrence.
Liu et al. [26] demonstrated that PDAI3 activates p-STAT3 in clear cell renal carcinoma. Up-regulation of PDIA3 improved clear cell renal cell carcinoma (ccRCC) cell survival by promoting a STAT3/ILF3 feedback loop. These findings provide a therapeutic target to treat ccRCC. Furthermore, Kondoa et al. [8]. illustrated that PDIA3 is closely linked with unfavourable phenotype of hepatocellular carcinoma through its association with STAT3 signalling. Knockdown of PDIA3 decreases p-STAT3 and downstream proteins of the STAT3 pathway.
Conclusions
We concluded that high expression of PDIA3 and p-STAT3 is related to highly aggressive cervical carcinoma with poor prognosis and high risk of recurrence after the standardised protocol of treatment. So, both PDIA3 and p-STAT3 could be novel biomarkers for tumour progression and a promising target in the management of cervical carcinoma patients.








