Introduction
Allergic conjunctivitis is reported to affect up to 15–20% of worldwide population [1–3]. Activation of H1 and H2 histamine receptors results in ocular itching and vasodilatation, respectively [4–6]. Avoidance of allergens and lubricants is commonly used to treat allergic conjunctivitis. Anti-histaminics are able to control inflammation reaction, while mast cell stabilizers can prevent mast cell degranulation on exposure to allergens [7–9].
Alcaftadine and olopatadine have been commonly applied to treat allergic conjunctivitis. One study reported that alcaftadine eyedrops and olopatadine eyedrops showed a comparable ocular symptom score on 3 days and 7 days for patients with mild or moderate allergic conjunctivitis [10]. As a dual-acting agent, alcaftadine is an anti-histamine and mast-cell stabilizer with anti-inflammatory property, and relieves ocular itching by inverse agonistic effects on H1, H2, and H4 receptors [11]. Olopatadine hydrochloride is a selective H1-receptor antagonist and mast-cell stabilizer with additional anti-inflammatory effects [12].
It is important to compare the efficacy of alcaftadine with olopatadine for the treatment of allergic conjunctivitis. Several RCTs reported their efficacy to improve ocular symptom for these patients, but the results were conflicting [10, 13–18].
This meta-analysis of RCTs aimed to assess the efficacy and safety of alcaftadine versus olopatadine in patients with allergic conjunctivitis.
Material and methods
Study selection and data collection
This meta-analysis was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement and Cochrane Handbook for Systematic Reviews of Interventions [19, 20]. No ethical approval or patient consent was needed because the meta-analysis was performed by using previously published studies.
PubMed, Embase, Web of Science, EBSCO and the Cochrane library were systematically searched up to June 2024, and we used the search terms “conjunctivitis” AND “olopatadine” AND “alcaftadine”. The inclusion criteria included: (1) study design was RCT; (2) patients had allergic conjunctivitis; (3) intervention treatments were alcaftadine versus olopatadine. Patients with hypersensitivity to the study medications were excluded.
Quality assessment
Methodological quality of individual RCTs was assessed by using the Jadad Scale [21], which included three evaluation elements: randomization (0–2 points), blinding (0–2 points), dropouts and withdrawals (0–1 points). The score of Jadad Scale varied from 0 to 5 points. Jadad score ≤ 2 suggested low quality, while Jadad score ≥ 3 indicated high quality [22].
Outcome measures
We extracted the following information from the eligible RCTs: the first author, publication year, sample size, age, weight, male, ocular symptom score and drug methods. The primary outcomes were the ocular symptom score on 3 days, 7 days, and 14 days. Secondary outcomes included the conjunctival hyperaemia score on 14 days and adverse events.
Statistical analysis
Mean difference (MD) with 95% confidence interval (CI) was used to evaluate continuous outcomes and odds ratio (OR) with 95% CI was used to assess dichotomous outcomes. I 2 statistic was used to assess the heterogeneity, and I 2 > 50% indicated significant heterogeneity [23]. The random-effect model was used for the significant heterogeneity, and otherwise the fixed-effect model was used. We conducted the sensitivity analysis through detecting the influence of a single study on the overall estimate via omitting one study in turn or using the subgroup analysis. Statistical analyses were performed by three authors. P < 0.05 indicated statistical significance and Review Manager Version 5.3 was used in all statistical analyses.
Results
Literature search, study characteristics and quality assessment
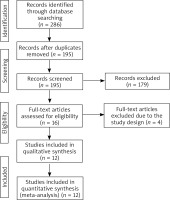
Figure 1 demonstrated the flow chart for the selection process and detailed identification. 286 publications were searched after the initial search of databases. 97 duplicates and 179 papers after checking the titles/abstracts were excluded. Four studies were removed because of the study design and twelve RCTs were included in the meta-analysis [10, 13–18, 24–27].
Table 1 showed the baseline characteristics of twelve eligible RCTs. The twelve studies were published between 2013 and 2022, and total sample size was 1064. Alcaftadine was administered at a concentration of 0.25%, while olopatadine was administered at a concentration of 0.1% or 0.2%. Among the twelve RCTs, five studies reported the ocular symptom score on 3 days [10, 13, 15, 16, 18], six studies reported the ocular symptom score on 7 days and 14 days [10, 13, 15–18], five studies reported the conjunctival hyperaemia score on 14 days [10, 13, 15, 16, 18], while four studies reported adverse events [14, 17, 18, 26]. Jadad scores of the included studies varied from 3 to 4, and thus all studies had high quality based on quality assessment.
Table 1
Characteristics of included studies
Primary outcomes: ocular symptom score on 3, 7 and 14 days
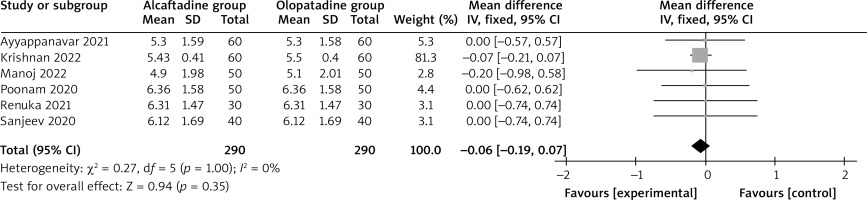
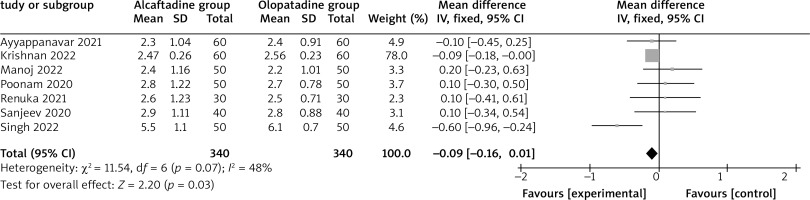
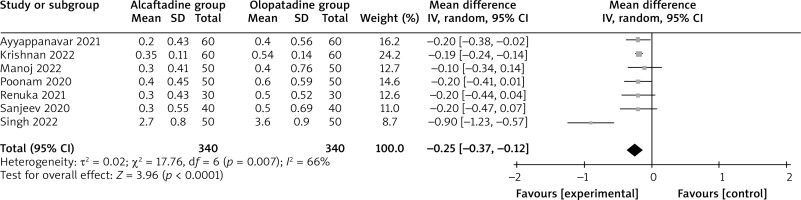
Compared with olopatadine for the treatment of allergic conjunctivitis, alcaftadine showed a comparable ocular symptom score on 3 days (MD = –0.06; 95% CI = –0.19 to 0.07; p = 0.35) with no heterogeneity among the studies (I 2 = 0%, heterogeneity p = 1.00, Figure 2), but was able to significantly decrease the ocular symptom score on 7 days (MD = –0.09; 95% CI = –0.16 to –0.01; p = 0.03) with low heterogeneity among the studies (I 2 = 0%, heterogeneity p = 0.87, Figure 3), and the ocular symptom score on 14 days (MD = –0.25; 95% CI = –0.37 to –0.12; p < 0.0001) with significant heterogeneity among the studies (I 2 = 66%, heterogeneity p = 0.007, Figure 4).
Sensitivity analysis
Significant heterogeneity was observed for the ocular symptom score on 14 days. As shown in Figure 4, the study conducted by Singh showed results that were almost out of range of the others and probably contributed to the heterogeneity [17]. After excluding this study, the results suggested that compared with olopatadine intervention for allergic conjunctivitis, alcaftadine intervention was still associated with decreased the ocular symptom score on 14 days (MD = –0.19; 95% CI = –0.23 to –0.15; p < 0.00001), and no heterogeneity remained (I 2 = 0, p = 0.99).
Secondary outcomes
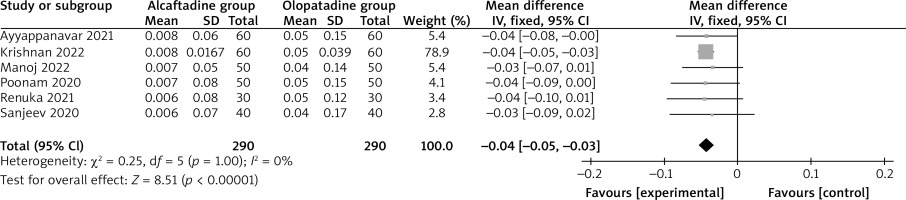
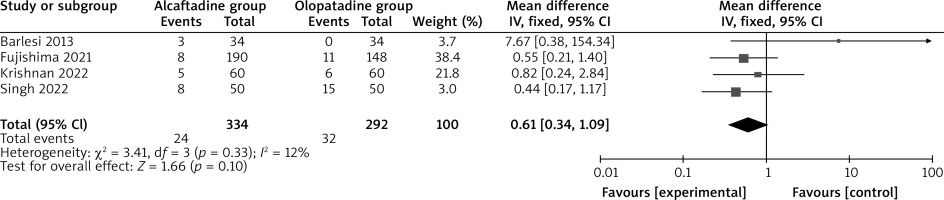
Compared with olopatadine intervention for allergic conjunctivitis, alcaftadine intervention decreased the conjunctival hyperemia score on 14 days (MD = –0.04; 95% CI = –0.05 to –0.03; p < 0.00001; Figure 5). Both alcaftadine and olopatadine had similar incidence of adverse events (OR = 0.61; 95% CI = 0.34 to 1.09; p = 0.10; Figure 6).
Discussion
Previous studies compared the efficacy of alcaftadine with olopatadine for allergic conjunctivitis, but the results were not well established. For instance, topical 0.25% alcaftadine eyedrops with topical 0.2% olopatadine eyedrops were used in 150 patients with allergic conjunctivitis. The results reported that alcaftadine were better to reduce the total ocular symptom score and conjunctival hyperaemia score than olopatadine for allergic conjunctivitis [13]. However, another study reported a similar ocular symptom score on 3 days and 7 days for patients with allergic conjunctivitis between the alcaftadine group and olopatadine group [10].
Considering these inconsistent results, twelve RCTs and 1064 patients diagnosed with allergic conjunctivitis were included in this meta-analysis, and the results found that compared with olopatadine, alcaftadine was able to remarkably decrease the ocular symptom score on 7 days, ocular symptom score on 14 days and conjunctival hyperaemia score on 14 days but demonstrated no effect on the ocular symptom score on 3 days. These indicated that alcaftadine was more effective than olopatadine to treat allergic conjunctivitis.
In terms of sensitivity analysis, significant heterogeneity remained for the ocular symptom score on 14 days. After excluding the study performed by Singh [17], no heterogeneity remained (I 2 = 0). Two factors may result in the significant heterogeneity. Firstly, allergic conjunctivitis had different levels of severity, which may affect the efficacy assessment. Secondly, olopatadine was administered at a concentration of 0.1% or 0.2%.
Alcaftadine and olopatadine hydrochloride became two commonly dual-acting antiallergic agents for allergic conjunctivitis, and they had the ability to inhibit histamine receptor activation directly and allergic responses by stabilizing mast cells indirectly [11, 28]. In the model with allergic conjunctivitis, alcaftadine is better to significantly inhibit eosinophil recruitment by a broad spectrum of H1, H2, and H4 histamine receptors antagonism and have a protective effect on epithelial tight junction protein expression in the conjunctiva than olopatadine hydrochloride [29]. Alcaftadine is able to protect epithelial tight junction protein markers from allergic inflammation-based degradation, but olopatadine failed to protect from degradation [30]. These supported the better efficacy of alcaftadine in relative to olopatadine for the treatment of allergic conjunctivitis based on the results of this meta-analysis.
The incidence of adverse events was similar between alcaftadine and olopatadine in this meta-analysis. 0.25% alcaftadine and 0.2% or 0.1% olopatadine were generally safe and mild for allergic conjunctivitis [13, 14, 17, 18, 31]. Several limitations should be taken into consideration. Firstly, our analysis was based on twelve RCTs and more studies with large patient samples were needed to confirm the results. Secondly, allergic conjunctivitis with various levels of severity were included in this meta-analysis, which may affect the results. Thirdly, there was significant heterogeneity, which may be caused by different concentrations of olopatadine.