Introduction
Gastric and gastroesophageal junction cancer (G/GEJC) presents a significant global health burden, with over 1,000,000 new cases reported in 2018 and substantial mortality rates [1]. This type of cancer ranks fifth in terms of frequency of diagnosis and is the third leading cause of cancer-related deaths globally. The rates of G/GEJC are notably higher in eastern Asia [1].
Immunotherapy (IT) has emerged as a potential treatment option, with programmed cell death ligand-1 (PD-L1) expression considered a biomarker for its effectiveness [2]. Two PD-L1 expression scoring systems have emerged: tumour proportion score (TPS) and combined positive scores (CPS). Tumour proportion score measures tumour cell PD-L1 expression, while CPS assesses PD-L1 expression in both tumour and immune cells. Both scores have been applied in pivotal gastric cancer (GC) clinical trials evaluating PD-1 inhibitor efficacy [3–5]. In the case of GC, CPS has been shown to be a more sensitive prognostic biomarker compared to TPS and is therefore more commonly used [6]. By measuring the expression of PD-L1 using CPS, it is possible to predict which individuals are likely to benefit from immune checkpoint inhibitors (ICIs) such as nivolumab and pembrolizumab [7]. Studies have shown that a significant proportion (ranging 25–65%) of patients with GC overexpress PD-L1, regardless of the scoring method used [8, 9]. As treatment options evolve, IT targeting PD-L1 expression has garnered attention as a potential therapeutic avenue for G/GEJC. However, the comparative efficacy of PD-L1 inhibitors versus chemotherapy in G/GEJC patients, particularly in relation to PD-L1 expression levels assessed through the CPS scoring method, remains a subject of uncertainty.
Immune checkpoint inhibitors such as programmed cell death 1 (PD-1) and PD-L1 inhibitors have been recommended as treatments for G/GEJC, which overexpress immune checkpoint ligands [9–11]. Pembrolizumab, in particular, has demonstrated antitumor activity in advanced G/GEJC patients who are PD-L1 positive [3, 5, 12]. Elevated expression of PD-L1 has been observed in up to 65% of G/GEJC cases and is associated with specific subtypes of GC and tumours with a high mutational burden [9–11, 13]. However, there is currently no consensus on the role of PD-L1 expression as a prognostic biomarker in advanced GC [14]. In Asian patients, a phase III trial showed that nivolumab, when administered as a third or later line of treatment, improved overall survival (OS) compared to placebo in unresectable advanced or recurrent GC that had progressed after chemotherapy. As a result, nivolumab has been approved for the treatment of this condition in Japan, Taiwan, and South Korea [4]. Pembrolizumab, based on data from the KEYNOTE-059 study, has been approved in the United States for the treatment of recurrent locally advanced or metastatic advanced G/GEJC patients expressing PD-L1 CPS ≥ 1, who have progressed on at least 2 previous lines of therapy [3]. However, in the KEYNOTE-061 trial, pembrolizumab did not show superiority to paclitaxel in second-line GC treatment [5]. Post hoc analysis revealed that the treatment effect was greater in patients with a PD-L1 CPS ≥ 10 than in those with CPS ≥ 1 [5]. While the combination of IT and chemotherapy has shown potential in G/GEJC treatment, further investigation is needed to determine the target population and the role of PD-L1 expression in patient selection and management. This may differ from the use of single-agent IT.
Thus, we performed a meta-analysis to evaluate the CPS and survival outcomes in G/GEJC after PD-L1 inhibitor versus chemotherapy.
Material and methods
Data sources and searches
A search was conducted using renowned databases such as PubMed, Google Scholar, and Web of Science from inception until 10 April 2023. Specific search terms and medical subject headings were used to identify relevant studies, as shown in Table 1. The search was limited to randomised control trials (RCTs) published in English language. Because the analyses utilised data from previously published research, no ethical approval or patient consent was required. The study is registered with PROSPERO under the registration number CRD42023495607.
Table 1
Search strategy
Selection criteria
The present study was performed in accordance with the PICOS criteria [15], which includes Population (P), Intervention (I), Comparison (C), and Outcome (O).
Population: patients with advanced G/GEJC characterised by CPS.
Intervention: ICIs, particularly PD-L1.
Comparison: chemotherapy treatment.
Outcome
The primary clinical endpoint analysed in this study was OS, and the secondary endpoint was progression-free survival (PFS). Overall survival was defined as the time from randomisation until death from any cause, while PFS was measured as the time from randomisation until disease progression or death from any cause.
Inclusion criteria:
The meta-analysis included clinical trials that involved patients diagnosed with histologically confirmed advanced G/GEJC;
Randomised phase III trials reported in English;
Studies that investigated CPS PD-L1 and chemotherapy were considered;
Survival data were required for inclusion;
Unresectable advanced, recurrent, or metastatic disease were accepted.
Exclusion criteria:
We excluded studies that are not original, systematic reviews, meta-analysis, case report, non-English papers, and animal studies;
Randomised control trials that were based on overlapping patients;
Papers with insufficient data were excluded;
Randomised control trials with ambiguous clinical outcomes were excluded.
Data extraction
Endnote 20 was used to remove duplicate entries. The titles and abstracts of the selected papers were evaluated by 2 impartial reviewers. A third reviewer was consulted to settle disagreements. First author details, publication year, trial type, age, clinical trial number, tumour type, research design, clinical trial phase, and interventions were acquired. Results such as CPS levels in patients receiving chemotherapy and PD-LI inhibitor were then gathered, as shown in Table 2. Table 3 displays the survival outcomes, which includes OS and PFS.
Table 2
Characteristics of selected studies
| Author | Year | Country | Study design | Clinical Trials. gov number | Tumour type | Age (years) Median (IQR) | Trial phase | CPS ≥ 1/≤ 1 in PD-L1 vs. chemotherapy | Number of patients (PD-L1/chemotherapy) |
|---|---|---|---|---|---|---|---|---|---|
| Wainberg et al. [21] | 2021 | USA | RCT | NCT02370498 | G/GEJC | 66 (35–79)/60 (37–76) | III | ≥ 1 : 196/199 | 296/296 |
| Kelly et al. [19] | 2021 | USA | RCT | NCT02743494 | G/GEJC | 62 (26–82)/61 (26–86) | III | ≥ 1 : 89/40 ≤ 1 : 374/196 | 532/262 |
| Bang et al. [20] | 2018 | South Korea | RCT | NCT02625623 | 59 (29–86)/61 (18–82) | III | ≥ 1 : 46/39 ≤ 1 : 111/121 | 185/186 | |
| Moehler et al. [18] | 2020 | Germany | RCT | NCT02625610 | G/GEJC | 62/61 | III | ≥ 1 : 30/24 ≤ 1 : 194/190 | 249/250 |
| Shitara et al. [12] | 2020 | Spain | RCT | NCT02494583 | G/GEJC | 61 (20–83)/62 (23–87) | III | - | 256/250 |
| Chao et al. [17] | 2021 | USA | RCT | NCT02370498 | G/GEJC | 67 (36–76)/63 (43–75) | III | ≥ 1 : 13/11 | 15/12 |
| Fuchs et al. [23] | 2021 | USA | RCT | NCT02370498 | G/GEJC | 62.5 (27–87) /60.0 (20–86) | III | ≤ 1 : 99/96 | 296/296 |
| Muro et al. [24] | 2023 | Japan | RCT | NCT02370498 | G/GEJC | 68 (38–75)/66 (27–77) | III | – | 27/38 |
| Huang et al. [22] | 2020 | China | RCT | NCT03099382 | G/GEJC | 60 (54–65)/60 (54–65) | ≥ 1 : 93/98 | 228/220 | |
| Kojima et al. [25] | 2020 | Japan | RCT | NCT02564263 | G/GEJC | 63 (28–84)/62 (24–84) | III | – | 314/314 |
Table 3
Survival rate according to programmed cell death ligand 1 combined positive scores
| Author | OS HR (95% CI) | PFS HR (95% CI) |
|---|---|---|
| Wainberg et al. [21] | – | 0.86 (0.56–1.33) |
| Kelly et al. [19] | 1.1 (0.9–1.4) | 1.73 (1.4–2.2) |
| Bang et al.[20] | 0.9 (0.74–1.11) | – |
| Moehler et al. [18] | 0.91 (0.69–1.18) | – |
| Shitara et al. [12] | 0.81 (0.66–1.00) | 1.25 (1.02–1.54) |
| Chao et al. [17] | 0.64 (0.41–1.02) | – |
| Fuchs et al. [23] | 0.67 (0.39–1.15) | 1.21 (0.69–2.13) |
| Muro et al. [24] | 0.67 (0.39–1.15) | 1.21 (0.69–2.13) |
| Huang et al.[22] | 0.71 (0.57–0.87) | – |
| Kojima et al. [25] | 0.69 (0.52–0.93) | – |
Publication bias
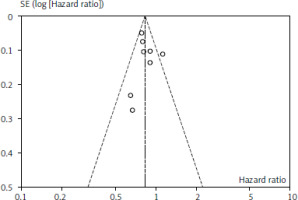
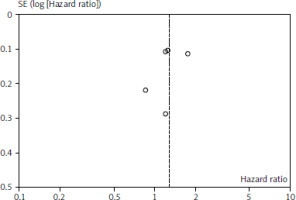
Figures 1 and 2 depict OS and PFS between PD-L1 inhibitor and chemotherapy funnel plot by log hazard ratio for the above-mentioned comparisons. These funnel plots show that most of the analysed studies are centred on the median axis, and that all of the studies fall within the funnel, indicating that there is no significant publication bias.
Quality assessment
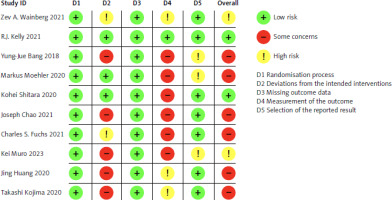
The risk of bias in each study was evaluated using the Cochrane Risk-of-Bias Tool for randomised trials (RoB 2.0). Studies were classified into 3 groups: low risk of bias, some concerns, or high risk of bias, based on 5 domains: randomisation process, intervention deviations, missing outcome data, outcome measurement, and selection of reported results. A study was deemed high risk if any domain was rated as such, and low risk if all domains were rated as low risk, as illustrated in Figure 3.
Statistical analysis
Statistical analysis was conducted using Review Manager version 5.4.1. We calculated odds ratios (ORs) along with 95% confidence intervals (CIs) for dichotomous data, employing a fixed effects model based on the Mantel- Haenszel statistical method. Survival data, such as OS and PFS, were assessed using the hazard ratios (HRs) and 95% CIs. To assess heterogeneity among the studies, we utilised the Q statistic and I2 statistic. Considerable variability was detected, as indicated by an I2 statistic above 50% [16]. We employed a fixed effects approach when I2 was 50% or less and a random effects approach when it exceeded 50%. Funnel plots were used to mitigate publication bias. Statistical significance was set at p < 0.05.
Results
Patients’ characteristics
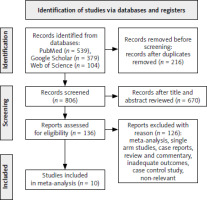
A total of 1022 studies were initially searched. After removing 216 duplicates, 806 records remained. Following a review of the titles and abstracts, 670 studies were selected for further evaluation. Out of these, 136 studies were assessed for eligibility. However, 126 studies were excluded for various reasons. Ultimately, 10 studies were included in the meta-analysis, including 4522 patients diagnosed with advance G/GEJC, as shown in Figure 4. Among these patients, 2398 were administered with PD-L1 inhibitor and 2124 received chemotherapy. The characteristics of the included studies are summarised in Table 1. All the trials were phase III. These studies took place in the USA, South Korea, Germany, Spain, Japan, and China.
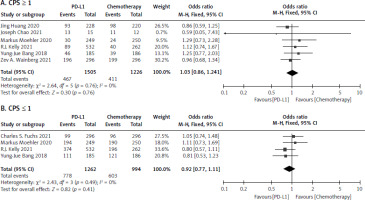
CPS ≥ 1 and CPS ≤ 1
Six studies [17–22] were collected for CPS ≥ 1 involving 1505 in the PD-L1 inhibitor and 1226 in the chemotherapy group. The analysis reported no statistically significant difference among the 2 treatment arms (I2 = 0.0%, p = 0.76 > 0.1). The OR for CPS ≥ 1 was 1.03 [95% CI: 0.86–1.24] as seen Figure 5 A. Four studies [18, 19, 20, 23], including 1262 patients in the PD-LI inhibitor, and 994 in the chemotherapy reported the OR of the CPS ≤ 1. Because there was no significant heterogeneity (I2 = 0.0%, p = 0.49 > 0.1), a meta-analysis was conducted using a fixed effects model. The OR of CPS ≤ 1 was 0.92 [95% CI: 0.77–1.11], indicating that there was no significant difference for CPS ≤ 1 between patients who had PD-L1 therapy and chemotherapy, as shown in Figure 5 B.
Survival outcomes among combined positive scores of patients (overall survival and progression-free survival)
Nine studies [12, 17–20, 22–25] were recorded for patients who were administered PD-L1 inhibitor and chemotherapy. The meta-analysis showed a significant difference for OS between the 2 treatment arms (HR 0.83, [95% CI: 0.78–0.88] and p < 0.00001]). The patients who were administered PD-L1 had higher OS as compared to chemotherapy, as seen in Figure 6 D. This benefit is probably due to the enhanced antitumour immune response and increased tumour cell killing mediated by PD-L1 inhibitors.
Fig. 6
Forest plot of survival outcomes between programmed cell death ligand-1 and chemotherapy no. overall survival (D), progression-free survival (E)
OS – overall survival, PFS – progression-free survival
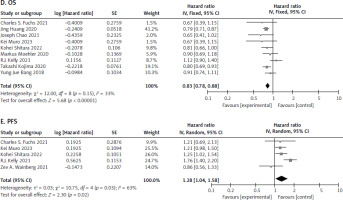
In addition, 5 studies [12, 19, 21, 23, 24] were extracted for PFS. The analysis showed significant difference between the 2 treatment groups (HR 1.28, [95% CI: 1.04–1.58], p = 0.02). The patients who received chemotherapy saw a better PFS as compared to PD-L1 therapy, as shown in Figure 6 E. This may be attributed to the immediate cytotoxic effects of chemotherapy, which can rapidly reduce tumour burden, whereas PD-L1 inhibitors may take longer to exert their immunomodulatory effects.
Discussion
Gastric and gastroesophageal junction cancer is biologically diverse, which makes treatment more challenging. A previous study attempted to determine the appropriate cutoff value for PD-L1 expression in IT for GC [26]. Research has shown that ICI-based therapy can extend survival in patients with melanoma or non-small cell lung cancer across all age groups [27].
The association between PD-L1 expression levels and the effectiveness of ICIs inhibitors has been a topic of discussion. Some studies suggest that patients with low PD-L1 expression may not benefit from IT, while others demonstrate that all treated patients with advanced G/GEJC experience significant improvements in OS and PFS, regardless of their PD-L1 expression levels [7]. However, conflicting findings exist, and a recent RCT indicated that PD-L1 expression levels are associated with OS [28, 29]. Furthermore, a review of 15 studies found inconsistent results regarding the prognostic significance of PD-L1 expression on the CPS for OS in G/GEJC patients [29]. Some studies did not find a strong link between prognosis and PD-L1 expression in G/GEJC patients [9]. Interestingly, our meta-analysis found no significant differences in CPS values between patients treated with PD-L1 inhibitors and those receiving chemotherapy. This finding brings up essential points: although CPS is a well-recognised marker for predicting treatment outcomes in cancers like head and neck squamous cell carcinoma and gastric carcinoma, supported by studies such as KEYNOTE-012 and KEYNOTE-055, our results suggest that CPS may not reliably indicate treatment response in patients with advanced G/GEJC [30, 31]. Consequently, it is important for clinicians to take a comprehensive approach that considers a range of factors beyond CPS when making treatment decisions, ensuring that they account for clinical significance to optimise patient outcomes.
For survival analysis, the present study demonstrated a significant difference in OS between the 2 treatment arms (HR 0.83, [95% CI: 0.78–0.88], p < 0.00001). The program death ligand-1inhibitor showed superior OS compared to the chemotherapy group. However, in PFS analysis, the results favoured chemotherapy over PD-L1 inhibitor treatment. The difference in OS and PFS results may be due to PD-L1 inhibitors having a delayed treatment effect, improving OS but not PFS. Specifically, PD-L1 inhibitors enhance the immune response, taking time to manifest clinically, and they reduce mortality risk by slowing disease progression without necessarily preventing initial progression. This leads to improved OS, but not necessarily PFS, whereas chemotherapy has a more immediate impact on reducing tumour burden, resulting in improved PFS but potentially lacking the long-term survival benefits conferred by PD-L1 inhibitors. Consistent with our findings, a separate meta-analysis indicated that anti-PD-1/PD-L1 therapy significantly outperformed chemotherapy alone in terms of OS (OR = 0.66, 95% CI: 0.47–0.92, p = 0.02). However, the difference in PFS did not reach statistical significance (OR = 0.93, 95% CI: 0.62–1.39, p = 0.72) [32]. While single-agent PD-L1 inhibitors were observed to improve OS relative to chemotherapy in advanced G/GEJC patients, our study also indicates that PD-L1 antagonists may not outperform chemotherapy overall [33, 34]. Specifically, among patients with PD-L1 CPS ≥ 1, single-agent IT led to prolonged OS (HR = 0.84, 95% CI: 0.74–0.96) but did not improve PFS (HR = 1.38, 95% CI: 0.91–2.09), a finding also supported by our meta-analysis [26]. Interestingly, another study reported that G/GEJC patients treated with PD-L1 inhibitors demonstrated better PFS compared to those treated with chemotherapy [33]. In trials of ICIs combined with chemotherapy versus chemotherapy alone in patients with a PD-L1 CPS score of 1–4, no significant differences in OS (0.950 (95% CI: 0.747–1.209, p = 0.678) and PFS (0.958 (95% CI: 0.743–1.236, p = 0.743) were observed [7]. The relationship between OS and PFS in clinical trials of ICIs varies depending on the specific disease and patient population studied [35]. The median OS with chemotherapy in G/GEJC patients with PD-L1 CPS ≥ 1 is consistent with previous global studies [36–39]. However, the OS benefit with PD-L1 inhibitors compared to chemotherapy did not directly correlate with PFS in patients with PD-L1 CPS ≥ 1, and a shorter PFS was observed with PD-L1 inhibitors [4, 31, 40]. Selected patients with CPS enrichment have shown long-term efficacy with PD-L1 inhibitors in GEJC [28, 41, 42]. Pembrolizumab, a PD-L1 inhibitor, showed improved safety but did not significantly enhance survival outcomes compared to chemotherapy in previous trials [3, 5, 12]. One possible explanation for the similar efficacy between PD-L1 inhibitors and chemotherapy in G/GEJC treatment could be the complex nature of the tumour microenvironment, characterised by immune system evasion and immunosuppressive mechanisms. Both PD-L1 inhibitors and chemotherapy can activate the immune system and potentially have anti-tumour effects, contributing to similar impacts on patient outcomes.
It is significant to remember that there are several limitations with this meta-analysis. Initially, there was a small sample size in the analysis, which raised the possibility of bias. To produce stronger proof, further research with bigger sample sizes is required. Secondly, there might have been differences in patient characteristics, treatment plans, and follow-up periods across the studies that were part of this study, which could have affected the results. Due to inadequate data, subgroup analysis based on these criteria were not feasible. Furthermore, it is possible that the included studies’ follow-up periods were not long enough to reliably record long-term survival results. Long-term studies would be beneficial in evaluating the enduring effects of treatment responses and possible distinctions between chemotherapy and PD-L1. Furthermore, because these factors may affect the course of treatment, it is critical to take into account the unique characteristics of each patient, including PD-L1 expression levels, tumour mutation load, and immune infiltration. Unfortunately, the small amount of data available meant that these variables could not be evaluated sufficiently in this meta-analysis. Notwithstanding these drawbacks, the meta-analysis adds to the corpus of knowledge already available about the effectiveness of PD-L1 and chemotherapy in patients with advanced G/GEJC who are CPS positive. Based on the results, it appears that both therapy approaches are reasonable choices for these patients’ care. However, treatment decisions should be individualised based on patient factors, tumour characteristics, and the availability of treatments.
Conclusions
This study demonstrates that PD-L1 inhibitors offer a sig-nificant survival benefit over chemotherapy in patients with advanced G/GEJC, particularly in terms of overall survival. However, chemotherapy provides better PFS outcomes. These findings suggest that PD-L1 inhibitors may be a preferred treatment option for patients with advanced G/GEJC, especially those with high CPS values, while chemotherapy may be more suitable for patients requiring rapid tumour control. Further research is needed to optimise treatment strategies and improve patient outcomes in this challenging disease setting.