Introduction
Colorectal cancer (CRC) is the third most common malignancy, with 1.4 million new diagnoses annually worldwide, and it is the fourth most common cause of cancer-related death [1]. Approximately 20% of CRC cases present with acute colonic obstruction, and this is more common in the left colon [2]. Morbidity and mortality rates are higher in emergent surgical procedures due to obstruction compared to elective surgery. Poor outcomes are considered to be associated with advanced stage, early recurrence, ischaemia, perforation, advanced age, fluid-electrolyte disorders, sepsis, and accompanying comorbidities [3–6].
Resection and anastomosis is the preferred procedure in obstructive proximal tumours, while treatment options are distinctive in obstructive distal tumours [7–9]. The advancements in postoperative supportive care and the development of specific colorectal surgery centres have improved emergent surgical outcomes. Studies on long-term oncologic outcomes in proximal and distal colon cancers undergoing emergent surgery are limited and heterogeneous [4, 10–14].
Aim
The purpose of this retrospective cohort study was to compare the early and long-term outcomes of emergent surgery in obstructive proximal and distal colorectal cancer at a single tertiary hospital.
Material and methods
Study population
Between January 2012 and June 2022, all consecutive patients who were admitted with acute obstructive colonic cancer in Dicle University Hospital emergency unit (Turkey) were included in the study. The general surgery and gastrointestinal surgical department in this institution deals with a wide array of surgical cases ranging from trauma surgery to hepatobiliary surgery or colorectal surgery. Approximately 80–100 cases of colorectal cancer are operated every year.
Data collection
Acute colonic obstruction was defined clinically (obstipation, abdominal distention, nausea, and vomiting) and confirmed by imaging (X-ray and abdominopelvic computed tomography scan with or without contrast). Patients who were operated within the first 24 h after admission from the emergency department and whose adenocarcinoma diagnosis was confirmed pathologically were included in the study. Non-adenocarcinoma pathology, follow-up with medical and supportive treatment, stenting, and occlusion site rectum were excluded. Demographic, clinical, American Society of Anesthesiologists (ASA) grade, Eastern Clinical Oncology Group performance status (ECOG PS), preoperative laboratory parameters (lactate dehydrogenase (LDH), albumin, carcinoembryonic antigen (CEA), and carbohydrate antigen 19–9 (CA 19–9)), and final pathology results of the patients were evaluated. The prognostic cutoff value for the LDH/albumin ratio (LAR) was taken as 5.27 [15]. The metastasis status was determined by clinical staging at diagnosis or in the postoperative period. Curative resection – R0 was considered completion of resection at initial diagnosis or in metastases with staged surgery.
Outcome measures and follow-up
Developing morbidity and mortality were reported according to Clavien-Dindo classification, and mortalities developing within the first 30 days were accepted as early [16]. Tumours localised to the colon from the cecum to the splenic flexure were defined as proximal, and tumours located to the distal part of the splenic flexure were defined as distal. According to tumour localisation, patients were divided into 2 groups and compared. Patients were routinely followed up with a protocol that included the following: every 3 months for the first 2 years, then every 6 months for the next 3 years, and then annually. Overall survival was defined as the period between the date of surgery and the date of death, whatever the cause. Disease-free survival – (in patients who did not develop early mortality and underwent curative surgery) was defined as the time (in months) to the date of the first postoperative relapse (local or distant).
Statistical analysis
Data were analysed using IBM SPSS Statistics for Windows ver. 21.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviations or median with ranges. Categorical variables were expressed as frequencies (number) and percentages (%). Continuous variables were compared using independent samples t-test, and categorical variables were compared using the χ2 test or Fisher exact test. The overall survival (OS) was estimated using the Kaplan-Meier method, and the outcomes were compared using the log-rank test. A Cox regression model was used to analyse the independent prognostic risk factors. Significant factors identified in univariate analysis were subsequently enrolled in the multivariate Cox proportional hazard model. A 2-tailed p level of < 0.05 was considered significant.
Results
From January 2012 to June 2022, 118 patients underwent emergent surgery for obstructed colon cancer (OCC). There were 46 (38.9%) patients in the proximal group and 72 (61.1%) patients in the distal group. The mean age was 58.7 ±17.4 years, and 53.4% of them were male. Curative surgery was performed in 42 (91.3%) patients in the proximal group and 59 (81.9%) patients in the distal group. Demographic, clinical, and laboratory characteristics of the 2 groups are summarised in Table I. The number of patients with LAR ≥ 5.27 was 32 (69.6%) and 65 (90.3%) in the proximal and distal groups, respectively, the difference was significant (p = 0.007). At the time of diagnosis, synchronous metastases were most frequently detected in the liver (16.9%) in both groups. During exploration, perforation was observed in 3 (6.5%) patients in the proximal group and 7 (9.7%) patients in the distal group. Single-session surgery including resection and anastomosis was performed on 31 (67.4%) and 29 (40.3%) patients in the proximal and distal groups, respectively, and the difference was statistically significant (p = 0.007).
Table I
Comparison of characteristics in obstructed proximal and distal colon cancers
[i] Values are presented as number (%) or mean ± standard deviation. ASA PS – American Society of Anesthesiologists physical status, ECOG PS – Eastern Cooperative Oncology Group performance status, CEA – carcinoembryonic antigen, CA 19-9, carbohydrate antigen 19-9, LDH – lactate dehydrogenase, NA – not available.
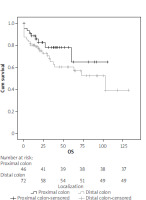
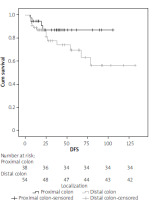
Postoperative clinical and pathological outcomes are shown in Table II. Early mortality was 8.7% in the proximal group and 12.5% in the distal group, and 13 (11.0%) patients died within the first 30 days. Early mortality was observed in 4 (20%) of 20 patients with synchronous liver metastases and 9 (9.2%) of 98 nonmetastatic patients (p = 0.231). Major complications (grade ≥ 3) were observed at a rate of 23% in both groups, and the length of hospital stay was similar (9.2 and 8.4 days). TNM stage distributions were similar, but the number of harvesting lymph nodes was 33.3 ±18.7 and 23.4 ±16.8 in the proximal and distal groups, respectively, and the difference was significant (p < 0.001). The median follow-up period was 26.2 months (interquartile range: 0–132). Five-year OS was 80.4% and 68.1% in the proximal and distal groups, respectively, which was better in the proximal group but not statistically significant (p = 0.152, Figure 1). In the proximal (38 patients) and distal (54 patients) groups who underwent curative resection and did not develop early mortality, 3-year DFS was 89.5% and 81.5%, respectively, and the difference was not significant (p = 0.165, Figure 2). Metastatic lymph node and advanced TNM stage (≥ III) were found to be negative prognostic factors in univariate analysis but were not significant in multivariate analysis. Tumour localisation was not a prognostic factor in univariate or multivariate analyses (p = 0.100, Table III).
Table II
Postoperative clinicopathological outcomes
Table III
Univariate and multivariate Cox regression analysis of survival outcomes in patients with obstructed proximal and distal colon cancers
Discussion
In this retrospective cohort study, it was determined that the early mortality and synchronous metastasis rates were high in obstructed colon cancers requiring emergent surgery, and 14.4% of curative surgery could not be performed. However, if R0 resection is achieved, oncological results are significantly improved and tumour localisation is not effective on OS and DSF. Behaviours of CRCs are heterogeneous, with different molecular carcinogenesis affecting their progression and metastasis (cellular hierarchy, tumour microenvironment, and clonal diversity, etc.) is a widely accepted pathway today [17]. TNM stage, lymphovascular invasion, perineural invasion, tumour differentiation, tumour marker level, localisation, nonelective surgery, obstruction, perforation, and anastomotic leakage are predictors that have an impact on prognosis [18]. Despite the slow growth pattern, approximately 20% of colon cancer cases are taken to emergent surgery because of obstruction. Advanced age, comorbidities, fluid-electrolyte disorders, sepsis, and advanced disease are more common in OCC, thus increasing morbidity and mortality rates compared to elective surgery [4–6, 19, 20]. In the presence of perforation with obstruction, early mortality rates are reported in a wide range (1.3–44%), and adjuvant chemotherapy is recommended because of adverse oncological outcomes [21–24]. Morbidity and mortality are significantly reduced in emergent surgeries performed by specific colorectal surgery teams [10, 11, 14, 22, 23]. Early mortality developed in 13 (11%) patients in our study, and it seems to be high when compared to the results of specialised centres.
It is accepted that the localisation of colon cancer has potentially different biological features and also affects the prognosis. The clinical picture is also different: iron deficiency anaemia due to slow blood loss and fatigue are common in proximally localised patients. Haematochezia and changes in bowel habits are more common in distal tumours [25]. A recent meta-analysis of 66 studies with over 1.4 million patients found that left colon cancer was associated with an increased survival rate compared to right colon cancer. The studies in this meta-analysis are heterogeneous, and studies involving emergent surgery for obstruction are very limited. In addition, 29 studies found that tumour localisation that did not affect survival was not included in the meta-analysis [26]. Manceau et al. [27] found that proximal tumour localisation was associated with poor prognosis in a study involving more than 2000 patients operated for OCC. In another study of the same team involving the same patient population, poor early and long-term clinical outcomes were found in proximal tumours [13]. However, in these 2 studies, the fact that the patients in the proximal tumour group were older, had higher ASA scores and comorbidities, and were at a more advanced stage at the time of diagnosis may be responsible for poor early and long-term outcomes. There are limited studies that have found similar long-term oncological outcomes in curative surgery for OCC [11, 12]. In a large series of 377 patients, Frago et al. [11] reported that 3-year survival for both groups was over 90% at stage I-II and over 70% at stage III and was similar. In this study, 3-year DFS (for stage III) was similar in the proximal and distal groups at 70.5% and 75.1%, respectively (p = 0.980). In the limited case study of Faucheron et al. [12], no significant difference was found in survival for proximal and distal OCC. In our study, the 5-year survival (for all stages) of the proximal and distal groups were 80.4% and 68.1%, respectively, and were similar (p = 0.152). In the analysis that included patients who underwent R0 resection and did not develop early mortality, 3-year DFS was similar in the proximal and distal groups, at 89.5% and 81.5%, respectively (p = 0.152). Oncological results were more favourable in the proximal tumour group, but the difference was not statistically significant. The mean age in our study is lower than in other studies, and ECOG PS 0-I dominant and adjuvant chemotherapy may contribute to our oncological results. In addition, since 2012, complete mesocolic excision for right and left colon cancer is performed in accordance with the principles defined by Hohenberger. We believe that this situation contributed to the higher number of lymph nodes dissected in the proximal group (33.3 ±18.7 vs. 23.4 ±16.8, p = 0.007) and to the improvement of oncological results.
There are some limitations of this study: its retrospective nature, low number of cases, and short follow-up period are the prominent ones. However, we think that it is important because of the limited number of studies in the literature reporting long-term oncological outcomes and comparing obstructed proximal and distal colon cancers.
Conclusions
Early mortality rates are still high in proximal and distal colon tumours causing obstruction and undergoing emergent surgery. If R0 resection is achieved, it is seen that oncological results are good and tumour localisation does not affect survival. We believe that surgical teams should focus on clinical applications targeting curative surgery and reducing early postoperative mortality.