Introduction
Portal hypertension is associated with increased portal venous pressure in the presence or absence of cirrhosis. The formation of portosystemic channels is a cardinal feature in portal hypertension, associated with the development of esophageal and gastric varices [1]. Varices are present in up to 40% of patients with cirrhosis, increasing to 85% in patients with Child-Pugh class C cirrhosis [2]. Compared to esophageal varices, gastric varices are less common, being present in about 2-20% of patients with portal hypertension [3]. Bleeding from varices represents a major decompensating event in the natural history of patients with cirrhosis and portal hypertension, associated with mortality in up to 20% at 6 weeks [4]. Bleeding from gastric varices is known to occur in 16%, 36%, and 44% at follow-up over 1, 3, and 5 years respectively [5]. Among the gastric varices, gastroesophageal varices type 1 (GOV1) is the most common (70%), followed by GOV2 (21%) and isolated gastric varices type 1 (IGV1). The risk of bleeding on the other hand is highest for IGV1 followed by GOV2 [6]. Although less frequent, gastric varices are associated with more profuse bleeding with a higher transfusion requirement, rebleeding and death [7]. While clear guidelines are available for the management of esophageal variceal bleeding, there is a lack of consensus on the management of gastric variceal bleeding. Various therapies, endoscopic and radiological, are available for the management of gastric variceal bleeding. However, the choice of therapy has been a matter of debate. Endoscopic variceal obturation using cyanoacrylate (CYA) glue has been the standard therapy for gastric variceal bleeding, endorsed in the Baveno guidelines as well [8]. However, this technique is fraught with technical issues such as incomplete obturation of the varices, a high rate of glue embolization, and rebleeding [9]. For patients with recurrent gastric variceal bleeding, endoscopic ultrasound (EUS) guided glue with or without coil injection is increasingly becoming popular [10]. Radiological therapies such as balloon occluded retrograde transvenous obliteration (BRTO) and transjugular intrahepatic portosystemic shunt (TIPS) have also been used primarily as rescue therapy with emerging data on its role as primary therapy in a select subset of patients [11, 12]. The existing literature has a paucity of head-to-head trials comparing different endoscopic and radiological modalities for gastric variceal therapy. We conducted this network meta-analysis to compare the outcomes of different endoscopic and radiological modalities for the treatment of gastric varices.
Material and methods
This systematic review and network meta-analysis is reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Network MetaAnalyses (PRISMA-NMA) guidelines [13]. The network meta-analysis was registered with PROSPERO (CRD42021281814).
Information sources and search strategy
We searched MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL), and Science Direct from January 2000 to September 2021 for all relevant studies. Additionally, we searched the reference lists of all identified trials, guidelines, and reviews on the topic for relevant trials.
Study selection
The titles and abstracts of the retrieved search records were independently screened by two reviewers (SG and SS) for inclusion and exclusion criteria. The same two reviewers examined the full text of potentially eligible cited literature. Any disagreement was resolved through discussion. Studies included in this NMA were randomized controlled trials (RCTs) and prospective or retrospective comparative studies fulfilling the following PICO criteria: (a) Patients – Cirrhotic or non-cirrhotic adults with gastroesophageal or isolated gastric varices; (b) Intervention – eight endoscopic or radiological treatment options: BRTO, TIPS, endoscopic thrombin injection (THB), variceal band ligation (VBL), endoscopic CYA glue injection (END-G), EUS-guided CYA glue injection (EUS-G), EUS-guided coil injection alone (EUS-C) and EUS-guided coil and CYA glue injection (EUS-C+G); (c) Comparison – Other endoscopic or radiological intervention; (d) Outcomes – variceal obliteration, overall rebleeding, moderate-severe adverse events and overall mortality. We included all original articles as well as conference abstracts, as EUS-guided procedures are a relatively new treatment modality and there are only a few RCTs and comparative studies concerning EUS-guided procedures. There was no bar on language as long as study outcomes were mentioned in the text. Single-arm studies, case series with sample size < 10, case series, studies reporting management of isolated esophageal varices, and studies involving persons < 18 years of age were excluded from the analysis.
Data extraction
Data extraction was performed independently by two investigators (SG and SS), and discrepancies were resolved by discussion, referring back to the original article. Data collection was done under the following headings: study author and year, study design, population (cirrhotic vs. non-cirrhotic), type of gastric varices, type of intervention used and the comparator arm, rate of variceal obliteration, the total number of adverse events, and serious adverse events, follow-up duration and number of deaths during follow-up.
Outcomes
The key outcomes of the study were the rate of obliteration of gastric varices, rate of overall rebleeding, moderate-severe adverse events, and all-cause mortality. All these outcomes were studied for each treatment modality including comparative outcomes between the different treatment strategies.
Risk of bias in individual studies and confidence in cumulative evidence
The risk of bias was assessed by two reviewers (SG and SS) using the Cochrane Collaboration’s Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool for non-randomized studies [14]. To assess the risk of bias in randomized trials, we used the Cochrane Risk-of-Bias tool for randomized trials (RoB 2) [15]. The assessment of the certainty of the evidence for all evaluable outcomes was done using the Confidence in Network Meta-Analysis (CINeMA) web application and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for network meta-analysis [16, 17].
Statistical analysis
Network meta-analysis was performed using the Stata 17.0 software package (Stata Corp LP, College Station, TX, USA) using a Bayesian random effect model [18]. The comparative efficacy of any two treatments was modeled as a function of each treatment relative to the reference treatment (END-G in this study, as it is the most common method used worldwide and there are no placebo studies). Treatment estimates were calculated as odds ratios (ORs) for binary outcomes, along with their 95% confidence interval (CI). The 95% prediction interval (PrI) was estimated from the interval plot, which includes the 95% CI and the effect estimates of future studies, taking into account the full uncertainty around the intervention estimate [19]. The pooled ORs from the NMA were compared with corresponding ORs from a pair-wise meta-analysis of direct comparisons to assess the inconsistency between direct and indirect comparisons. The global Wald test was performed to assess for global inconsistency. Inconsistency plots were created for the assessment of agreement of direct and indirect evidence [20]. Relative ranking of interventions for various outcomes was calculated as their surface under the cumulative ranking curve (SUCRA) [20]. Publication bias was assessed by examining the funnel plot asymmetry [21].
Results
A total of 4307 studies were identified from different electronic databases and manual searching. Figure 1 shows the flow diagram for study retrieval, identification, screening, and inclusion as per the latest 2020 PRISMA guidelines. A total of 34 studies [22-55] (Table 1) were included in the final analysis with a total population of 2783 patients. The number of participants in each study ranged from 15 to 176. Of these studies, 10 were randomized controlled trials [22, 24, 26, 27, 30, 45, 47, 51, 52, 54] and 24 were non-randomized studies (2 prospective cohort studies [28, 36] and 22 retrospective comparative analyses [23, 25, 29, 31-35, 37-44, 46, 48-50, 53, 55]). Thirty studies were published full articles [22-31, 33-38, 39-43, 45-47, 49-54] and 4 were conference abstracts [32, 39, 44, 48]. The majority of the included studies concerned secondary prophylaxis of gastric varices, except for the studies by Romero-Castro et al. (secondary prophylaxis: 76.7%) [33], Bang et al. (secondary prophylaxis: 90%) [39], Lôbo et al. (secondary prophylaxis: 53.1%) [47], and Robles-Medranda (secondary prophylaxis: 93.3%) [52], in which indications were both primary and secondary.
Fig. 1
Flow diagram for study retrieval and identification for network meta- analysis as per the PRISMA 2020 statements
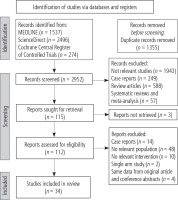
Table 1
Characteristics of the included studies
| Author, year | Country | Design, publication | No. of patients | Male/female | Etiology | Gastric varices classification | Interventions | Child-Pugh class (A/B/C)/score | Follow-up duration (months) |
|---|---|---|---|---|---|---|---|---|---|
| Lo, 2001 [22] | China | RCT, full text | 60 | 46/14 | HBV, HCV, alcohol | GOV1, GOV2, IGV1 | END-G: 31, VBL: 29 | END-G: 8/16/7, VBL: 5/17/7 | 9-14 |
| Mahadeva, 2003 [23] | UK | Retrospective, full text | 43 | 28/15 | Alcohol, cholestatic | GOV1, GOV2 | END-G: 23, TIPS: 20 | END-G: 4/8/9, TIPS: 4/6/10 | 6 |
| Choi, 2003 [24] | Korea | RCT, full text | 15 | 11/4 | Viral, alcohol | – | BRTO: 8, TIPS: 7 | – | 6-21 |
| Ninoi, 2004 [25] | Japan | Retrospective, full text | 104 | 61/43 | Viral, alcohol | – | BRTO: 77, TIPS: 27 | BRTO: 46/29/2, TIPS: 15/11/1 | TIPS: 41.2 ±32.4, BRTO: 26.9 ±16.5 |
| Tan, 2006 [26] | Taiwan | RCT, full text | 97 | 69/28 | Viral, mixed, alcohol | GOV1, GOV2, IGV1 | END-G: 49, VBL: 48 | END-G: 13/26/10, VBL: 12/25/11 | END-G: 56.7 ±59.2, VBL: 50.88 ±50.25 |
| Lo, 2007 [27] | Taiwan | RCT, full text | 72 | 53/19 | HBV, HCV, alcohol | GOV1, GOV2 | END-G: 37, TIPS: 35 | END-G: 12/19/6, TIPS: 9/20/6 | END-G: 32, TIPS: 33 |
| Hong, 2009 [28] | Korea | Prospective, full text | 27 | 21/6 | HBV, alcohol, HCV | GOV2, IGV1 | END-G: 14, BRTO: 13 | END-G: 3/8/3, BRTO: 5/6/2 | 1-38 |
| Procaccini, 2009 [29] | USA | Retrospective, full text | 105 | 69/36 | Alcohol, viral, NASH | – | END-G: 61, TIPS: 44 | – | END-G: 73.9, TIPS: 47.8 |
| El Amin, 2010 [30] | Egypt | RCT, full text | 150 | 108/42 | HCV, HBV | – | END-G: 75, VBL: 75 | END-G: 15/32/28, VBL: 20/40/15* | 6 |
| Min, 2011 [31] | Korea | Retrospective, full text | 103 | 84/19 | Alcohol, HBV, HCV | GOV1, GOV2, IGV1 | END-G: 52, BRTO: 15, VBL: 36 | END-G: 13/31/8, BRTO: 4/8/3, VBL: 9/21/6 | 65.13 |
| Lee YJ, 2012 [32] | Korea | Retrospective, abstract | 100 | – | – | – | BRTO: 68, TIPS: 32 | NS difference | – |
| Castro, 2013 [33] | Spain | Retrospective, full text | 30 | 22/8 | Alcohol, viral | IGV1, GOV2, GOV1 | END-G: 19, EUS-C: 11 | END-G: 6/6/7 EUS-C: 4/7/0* | 17.2 ±1.8 |
| Hong, 2013 [34] | Korea | Retrospective, full text | 84 | 73/11 | Alcohol, HBV, HCV | GOV1 | END-G: 64, VBL: 20 | END-G: 37/23/4, VBL: 8/10/2 | 38.13 |
| Lo, 2013 [35] | Taiwan | Retrospective, full text | 162 | 140/22 | HBV, alcohol, HCV | – | END-G: 118, VBL: 44 | END-G: 26/40/42, VBL: 10/15/19 | 1.4 |
| Tantau, 2014 [36] | Romania | Prospective, Full text | 37 | 21/16 | Viral, alcohol | GOV1, GOV2 | END-G: 19, VBL: 18 | END-G: 3/11/5, VBL: 8/7/3 | END-G: 14.04 ±7.04, VBL: 13.35 ±7.01 |
| Sabri, 2014 [37] | USA | Retrospective, full text | 50 | 29/21 | Alcohol, HCV, CGC | – | BRTO: 23, TIPS: 27 | – | 1-52 |
| Emori, 2014 [38] | Japan | Retrospective, full text | 112 | 64/48 | HCV, alcohol, HBV | IGV1 | END-G: 63, BRTO: 49 | END-G: 8.4 ±1.8, BRTO: 7.7 ±1.9 | 42.2 ±34.7 |
| Bang, 2015 [39] | USA | Retrospective, abstract | 71 | 46/25 | NASH, HCV, alcohol | GOV2, IGV1, GOV1 | END-G: 40, EUS-G: 31 | END-G: 95.0% cirrhotics, EUS-G: 83.9% cirrhotics | END-G: 12.0, EUS-G: 7.9 |
| Kochar, 2015 [40] | USA | Retrospective, full text | 169 | 105/64 | Alcohol, HCV, HBV | – | END-G: 29, TIPS: 140 | END-G: 11/10/7, TIPS: 37/64/24 | 1 |
| Kim, 2017 [41] | Korea | Retrospective, full text | 52 | 28/24 | Alcohol, viral, NASH | – | BRTO: 25, TIPS: 27 | – | BRTO: 2.9, TIPS: 2.1 |
| Lee SJ, 2017 [42] | Korea | Retrospective, full text | 142 | 115/27 | Alcohol, HBV, HCV | GOV2, IGV1, GOV1 | BRTO: 95, TIPS: 47 | BRTO: 6.6 ±1.6, TIPS: 7.4 ±1.6 | 28.2 ±28.3 |
| Gimm, 2018 [43] | Korea | Retrospective, full text | 176 | 139/37 | HBV, alcohol, HCV | GOV2, IGV1, IGV2 | BRTO: 157, TIPS: 19 | BRTO: 8.0 ±2.0, TIPS: 7.3 ±2.0 | – |
| Krill, 2018 [44] | USA | Retrospective, abstract | 28 | 20/8 | – | – | EUS-C: 6, EUS-G: 10, EUS-C+G: 12 | – | 1-4 |
| Hassan, 2018 [45] | Pakistan | RCT, full text | 60 | 40/20 | HCV, HBV | – | END-G: 30, VBL: 30 | END-G: 8/15/7, VBL: 6/17/7 | 24 |
| Bick, 2018 [46] | USA | Retrospective, full text | 104 | 62/42 | NASH, HCV, alcohol | GOV2, IGV1, GOV1 | END-G: 40, EUS-G: 64 | END-G: 95.0% cirrhotics, EUS-G: 84.4% cirrhotics | END-G: 16.3, EUS-G: 6.5 |
| Lobo, 2019 [47] | Brazil | RCT, full text | 32 | 13/19 | CGC, alcohol, HCV, | GOV2, IGV1 | END-G: 16, EUS-C+G: 16 | END-G: 13/3/0, EUS-C+G: 12/4/0 | 10 |
| Wang, 2019 [48] | USA | Retrospective, abstract | 56 | 36/20 | – | – | END-G: 14, EUS-C: 10, BRTO: 13, TIPS: 19 | – | 6 |
| Stein, 2019 [49] | USA | Retrospective, full text | 161 | 57/104 | NCPH: 10.5% | IGV1, GOV2 | END-G: 90, BRTO: 71 | Mean 8.1 in both groups | END-G: 17.3, BRTO: 12.8 |
| Bazarbashi, 2020 [50] | USA | Retrospective, full text | 40 | 27/13 | Alcohol, NASH, HCV | IGV1, GOV2, GOV1 | END-G: 30, EUS-C: 10 | END-G: 7/18/5 EUS-C: 3/4/1 | 6 |
| Lo, 2020 [51] | Taiwan | RCT, full text | 68 | 51/17 | Alcohol, HCV, HBV | GOV1, GOV2, IGV1 | END-G: 35, THB: 33 | END-G: 15/11/9, THB: 18/11/4 | 1.5 |
| Medranda, 2020 [52] | Ecuador | RCT, full text | 60 | 35/25 | NASH, alcohol | GOV2, IGV1 | EUS-C: 30, EUS-C+G: 30 | EUS-C: 26/3/1, EUS-C+G: 28/2/0 | 12 |
| Medranda, 2021 [53] | Ecuador | Retrospective, full text | 36 | 20/16 | – | GOV2, IGV1 | END-G: 19, EUS-C+G: 17 | – | 6 |
| Luo, 2021 [54] | China | RCT, full text | 64 | 39/25 | HBV, alcohol | GOV2, IGV1 | END-G: 32, BRTO: 32 | END-G: 12/14/6, BRTO: 17/9/6 | END-G: 27.1 ±12, BRTO: 27.6 ±14.3 |
| Choe, 2021 [55] | Korea | Retrospective, full text | 113 | 82/31 | HBV, alcohol, HCV | GOV1, IGV1, GOV2 | END-G: 72, BRTO: 41 | END-G: 32/35/5, BRTO: 25/14/2 | END-G: 36, BRTO: 34 |
[i] RCT – randomized controlled trials, HBV – hepatitis B virus, HCV – hepatitis C virus, NASH – non-alcoholic steatohepatitis, CGC – cryptogenic cirrhosis, GOV – gastroesophageal varices, IGV – isolated gastric varices, END-G – endoscopic glue injection, BRTO – balloon occluded retrograde transvenous obliteration, EUS-C – EUS-guided coil injection, EUS-G – EUS-guided glue injection, EUS-C+G – EUS-guided coil + glue injection, TIPS – transjugular intrahepatic portosystemic shunt, VBL – variceal band ligation, THB – endoscopic thrombin injection, VO – variceal obliteration, RB – overall rebleeding, AE – moderate-severe adverse events, MO – all-cause mortality
Risk of bias
Among the 10 RCTs, only 4 studies [26, 27, 47, 51] had low risk of bias and 6 RCTs had moderate [22, 30, 45, 52, 54] and 1 RCT had high risk of bias [24]. Among the 24 non-randomized studies, only 3 studies [36, 50, 55] had moderate risk of bias, while 20 studies had high risk of bias [23, 24, 28, 29, 31-35, 37-44, 48, 49, 53].
Network consistency
The network plot of individual interventions for all outcomes is summarized in Figure 2. There was neither any significant difference in the magnitude of pair-wise and network estimates (Tables 2 and 3) nor any significant loop-specific inconsistency between direct and indirect evidence. There was also no evidence of global inconsistency for any of the outcomes.
Fig. 2
Network plots for number of A) patients with gastric variceal obliteration, B) patients with overall rebleeding, C) patients with moderate-severe adverse events (AEs), D) patients with all-cause mortality
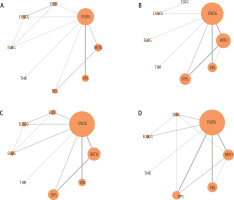
Table 2
League table of pairwise comparisons for the odds ratio of overall rebleeding.
[i] Treatment estimates are provided as the odds ratio with 95% confidence intervals (read from left to right). Significant pairwise comparisons are highlighted in bold. Direct estimates are positioned above the strategy labels and network estimates are positioned below the strategy labels. NA indicates that no studies made direct comparisons between treatments. NA – not applicable, BRTO – balloon occluded retrograde transvenous obliteration, END-G – endoscopic glue injection, EUS-G – EUS-guided glue injection alone, EUS-C – EUS-guided coil injection alone, EUS-C+G – EUS-guided coil and glue injection, TIPS – transjugular intrahepatic portosystemic shunt, THB – endoscopic thrombin injection, VBL – variceal band ligation.
Table 3
League table of pairwise comparisons for the odds ratio of overall mortality
[i] Treatment estimates are provided as the odds ratio with 95% confidence intervals (read from left to right). Significant pairwise comparisons are highlighted in bold. Direct estimates are positioned above the strategy labels and network estimates are positioned below the strategy labels. NA indicates that no studies made direct comparisons between treatments.
Outcomes
Number of patients with variceal obliteration
Eighteen studies [22, 24-28, 30, 33, 36, 41, 44-47, 51, 52, 54, 55] including 1141 patients reported data on gastric variceal obliteration from 8 interventions (Fig. 2A). THB (OR = 0.03, 95% CI: 0.00-0.39), EUS-C (OR = 0.05, 95% CI: 0.00-0.71), VBL (OR = 0.05, 95% CI: 0.01-0.22), TIPS (OR = 0.06, 95% CI: 0.02-0.20), and END-G (OR = 0.13, 95% CI: 0.04-0.43) were found to have lower odds of variceal obliteration compared to BRTO in network estimation. Compared to END-G, only VBL (OR = 0.40, 95% CI: 0.17-0.93) had a lower odds of variceal obliteration. On calculating the 95% PrI from the interval plot, only VBL (OR = 0.05, 95% PrI: 0.00-0.64) and TIPS (OR = 0.06, 95% PrI: 0.01-0.58) were found to have lower odds of variceal obliteration compared to BRTO. The global Wald test yielded a χ2 (4) = 1.73 and Prob > χ2 = 0.786, which indicates no evidence of inconsistency or heterogeneity.
The command <network rank max, all gen(prob)> was used to determine the ranking plot, as the treatment modality with maximum variceal obliteration will have highest probability. BRTO was ranked first (SUCRA 95.1) with the maximum probability of variceal obliteration. EUS-C+G was ranked the second-best modality (SUCRA 80.9) followed by END-G (SUCRA 54), EUS-G (SUCRA 53.7), TIPS (SUCRA 50.5), VBL (SUCRA 23.7), THB (SUCRA 21.3), and EUS-C (SUCRA 20.8) (Fig. 3A). The certainty of evidence for the SUCRA ranking was low.
Number of patients with overall rebleeding
Twenty-eight studies [22-32, 34-42, 45, 46, 49, 51-55] including 2404 patients reported data on overall rebleeding from 8 interventions for gastric varices. Compared to VBL, BRTO (OR = 0.10, 95% CI: 0.05-0.22), EUS-G (OR = 0.12, 95% CI: 0.04-0.34), TIPS (OR = 0.29, 95% CI: 0.13-0.65) and END-G (OR = 0.52, 95% CI: 0.31-0.87) had lower odds of overall rebleeding in network estimation. BRTO (OR = 0.19, 95% CI: 0.10-0.35) and EUS-G (OR = 0.23, 95% CI: 0.10-0.55) had lower odds of rebleeding compared to END-G, while BRTO (OR = 0.35, 95% CI: 0.19-0.64) had lower odds of rebleeding compared to TIPS (Table 1). The interval plot showed lower odds of rebleeding with BRTO (OR = 0.19, 95% PrI: 0.07-0.53) and EUS-G (OR = 0.23, 95% PrI: 0.07-0.78) compared to END-G and higher odds of rebleeding with BRTO (OR = 9.95, 95% PrI: 3.19-31.07), EUS-G (OR = 8.19, 95% PrI: 2.20-30.41) and TIPS (OR 3.50, 95%PrI 1.10–11.16) compared to VBL. The global Wald test yielded a chi2 (3) = 4.90 and Prob > chi2 = 0.179, which indicates no evidence of inconsistency or heterogeneity.
The command <network rank min, all gen(prob)> was used to determine the ranking plot, as the treatment modality with minimum overall rebleeding will have lowest probability. A SUCRA plot was generated from the ranking plot. Among all the compared treatment modalities, BRTO was ranked first (SUCRA 85.1) with the lowest probability of overall rebleeding. EUS-C+G was the second-best modality (SUCRA 78.8) followed by EUS-G (SUCRA 69.8), TIPS (SUCRA 50.2), END-G (SUCRA 37), EUS-C (SUCRA 32), THB (SUCRA 29.8), and VBL (SUCRA 17.2). The certainty of evidence for the SUCRA ranking was low.
Number of patients with moderate-severe adverse events
Twenty-nine studies [22, 23, 25-30, 32-39, 41-47, 49-52, 54, 55] including 2403 patients reported data on moderate-severe adverse events from 8 interventions for gastric varices. Reported moderate-severe adverse events included new onset ascites or worsening of ascites, pleural effusion, development of hepatic encephalopathy, pulmonary embolism, acute kidney injury, post-TIPS liver failure, splenic infarction, aspiration pneumonia, bacteremia/sepsis, portal vein thrombosis and hemoperitoneum. THB (OR = 0.02, 95% CI: 0.00-0.60), VBL (OR = 0.22, 95% CI: 0.08-0.58), EUS-G (OR = 0.23, 95% CI: 0.06-0.89), END-G (OR = 0.30, 95% CI: 0.15-0.60), and BRTO (OR = 0.32, 95% CI: 0.18-0.57) had lower odds of moderate-severe AEs compared to TIPS in network estimation. However, on analysis of the interval plot, the 95% PrI for all these comparisons included the null value (i.e., 1). The global Wald test yielded a χ2 (4) = 0.50 and Prob > χ2 = 0.9735, which indicates no evidence of inconsistency or heterogeneity.
Among the various treatment modalities, thrombin was ranked first (SUCRA 92.5) with the lowest probability of moderate-severe AE. EUS-C was the secondbest modality (SUCRA 66) followed by VBL (SUCRA 64.4), EUS-G (SUCRA 57.1), END-G (SUCRA 46.7), BRTO (SUCRA 39.1), EUS-C+G (SUCRA 30.3), and TIPS (SUCRA 3.7). The certainty of evidence for the SUCRA ranking was low.
Number of patients with mortality (all-cause)
Twenty-five studies [22-31, 34-36, 38, 40, 45, 47-55] including 2025 patients reported data on all-cause mortality from 7 interventions for gastric varices. In pairwise network estimation, BRTO had lower odds of mortality compared to END-G (OR = 0.49, 95% CI: 0.30-0.81), TIPS (OR = 0.39, 95% CI: 0.20-0.77) and VBL (OR = 0.40, 95% CI: 0.21-0.77) (Table 2). On the interval plot for all these comparisons, the null value was included in the 95% PrI. The global Wald test yielded a χ2 (7) = 10.19 and Prob > χ2 = 0.178, which indicates no evidence of inconsistency or heterogeneity.
Among the various treatment modalities, EUS-C+G was ranked first (SUCRA 73.5) with the lowest probability of overall mortality. TIPS was the secondbest modality (SUCRA 69.1) followed by thrombin (SUCRA 57), BRTO (SUCRA 53.7), EUS-C (SUCRA 49.3), VBL (SUCRA 24.2), and END-G (SUCRA 23.2). The certainty of evidence for the SUCRA ranking was low.
Publication bias
There was no evidence of publication bias, which was assessed qualitatively based on the symmetry in the funnel plot for all the studies reporting various outcomes.
The summary of findings for treatment comparisons with significant odds ratio for at least one outcome is shown in Table 4.
Table 4
Summary of findings table with comparison between interventions with significant odds ratio for at least one outcome.
* Risk of bias (–1); # Risk of bias (–2); ¥ Imprecision (–1); ¶ Heterogeneity (–1); ¢ Risk of bias (–1), Imprecision (–1); § Risk of bias (–2), Imprecision (–1);
∆ Risk of bias (–1), Incoherence (–1); ¤ Risk of bias (–2), Heterogeneity (–1); BRTO – balloon occluded retrograde transvenous obliteration, END-G – endoscopic glue injection, EUS-G – EUS-guided glue injection alone, EUS-C – EUS-guided coil injection alone, EUS-C+G – EUS-guided coil and glue injection, TIPS – transjugular intrahepatic portosystemic shunt, THB – endoscopic thrombin injection, VBL – variceal band ligation.
Discussion
Bleeding from gastric varices represents a life-threatening condition requiring immediate intervention with a significant risk of mortality. There has been an impasse as regards the choice of optimal intervention to ensure hemostasis and prevent rebleeding while assuring safety and averting mortality. Due to the lack of robust data, a consensus based on the management of esophageal varices has been extrapolated in a therapeutic approach towards gastric variceal bleeding. This is the first network meta-analysis comparing different endoscopic and radiologic interventions for the treatment of gastric varices in terms of variceal obliteration, rebleeding, adverse events, and mortality. BRTO was the best intervention in terms of obliteration and rebleeding followed by EUS-C+G. Endoscopic thrombin injection followed by EUS-guided coiling was the safest in terms of adverse events. EUS-C+G followed by TIPS showed the lowest probability of overall mortality.
After the first description of endoscopic therapy for gastric varices using glue injection in 1986 by Soehendra et al., over the years, it has become the gold standard therapy for obturation of gastric varices [56]. However, rebleeding is seen in 3.7% to 58% and may be early (due to incomplete obturation or extrusion of glue plug) or late (due to incomplete obturation, fresh collateralization, or recanalization of obturated varices). Assessment of adequacy of obturation is made by probing the injected varix for solidification. This technique is subjective and prone to erroneous interpretations, increasing the risk of rebleeding [57]. VBL is not routinely recommended for the management of gastric varices due to lower chances of obliteration and high rebleeding risk, especially in large varices, with difficulty using this technique in fundal varices (IGV-1 and GOV-2) [58, 59]. Considering various fallacies of these techniques, there is increasing use of endoscopic ultrasound and radiologic techniques for gastric variceal obturation.
Endoscopic ultrasound-guided coiling was first described by Binmoeller et al. [60]. EUS had the advantage of direct visualization of site of injection and use of Doppler to confirm flow obliteration. A recent meta-analysis by Mohan et al. assessed outcomes of EUS intervention for gastric varices and compared them with END-G [61]. Observational studies and abstracts were also included as part of the analysis. The rate of obliteration was higher with EUS than END-G (84% vs. 63%), but rates of pooled treatment efficacy (94% vs. 91%), early (7% vs. 5%), and late rebleeding (12% vs. 17%) were comparable. Another meta-analysis comparing EUS-C+G vs. monotherapy with EUS-G and EUS-C showed that EUS-C+G was better in terms of functional success and adverse events as compared to monotherapy [62]. In our meta-analysis, we found that EUS-C+G had a significant benefit as compared to END-G in terms of obliteration and rebleeding. EUS-C+G was the intervention with the lowest probability of overall mortality. However, the certainty of evidence remains low to very low, requiring further studies.
The choice between TIPS and BRTO may be guided by anatomy of the collateral system and also the presence of underlying complications of cirrhosis and portal hypertension. BRTO has often been criticized for post-procedural increased flow in the portal system based on the “throughput hypothesis”. TIPS on the other hand has often been criticized for not working as well for gastric varices as compared to esophageal varices based on the “proximity hypothesis” [63]. Whether a combination of these procedures works better than either is unclear. Wang et al. in their meta-analysis compared TIPS with BRTO for gastric varices [12]. BRTO showed mortality benefit over TIPS (RR = 0.81, 95% CI: 0.66-0.98) with a lower rate of rebleeding (RR = 2.61, 95% CI: 1.75-3.90). There was a higher rate of encephalopathy with TIPS, with higher although non-significant exacerbation of ascites with BRTO. In another meta-analysis by Paleti et al. [11], BRTO was associated with a lower rate of rebleeding (OR = 0.30, 95% CI: 0.18-0.48) and overall mortality (OR = 0.43, 95% CI: 0.21-0.87). The results of our meta-analysis are in agreement with the previous series with BRTO having the lowest probability of overall rebleeding. The probability of moderate-severe AEs was highest with TIPS followed by BRTO. However, unlike the previous meta-analysis, TIPS had the second-lowest probability of overall mortality, better than BRTO. This lower probability of overall mortality is likely due to the impact on portal hypertension and the natural history of other complications of cirrhosis.
The strengths of our systematic review include a thorough search of existing literature, with well-defined inclusion criteria, with the removal of potential bias by the inclusion of comparative studies only. Also, well-defined outcomes were studied with a low level of heterogeneity. The primary comparator in this systematic review was endoscopic glue injection, which has been the gold standard for gastric variceal bleeding. Our study has a few limitations. These include the limited number of studies with a small number of patients who underwent interventions apart from END-G. Also, subgroup analysis based on the severity of background cirrhosis could not be done. Our meta-analysis may not be representative of overall clinical practice wherein EUS-guided and radiologic interventions are performed at centers with expertise, making generalisability of these results difficult. While our meta-analysis included only comparative studies, few of the included studies were retrospective in nature, leading to possible selection bias. Data on the type and size of varix could not be compared, but most studies included cases of GOV2 and IGV1 for intervention. The efficacy of the combination of therapies such as END-G with TIPS or BRTO with TIPS could not be assessed. Lastly, rebleeding-related mortality is a more relevant end point than all-cause mortality, but such data were not available from the majority of the studies.
In conclusion, this is the first network meta-analysis of different endoscopic and radiologic therapies for the management of gastric varices. Considering the high rates of variceal obliteration, lower rates of bleeding, acceptable rate of adverse events, and lowest probability of mortality, EUS-C+G may be preferred over END-G in centers with expertise. BRTO may be a potential alternative to EUS-C+G considering high rates of obliteration, low rates of rebleeding, acceptable safety profile and higher certainty of evidence. TIPS, on the other hand, may be considered in patients with gastric variceal bleeding with other complications of cirrhosis, to mitigate the effect of clinically significant portal hypertension. Patient selection criteria for TIPS should be robust considering the higher chances of moderate-severe adverse events. There is potential for future large-scale comparative trials between EUS-guided and radiologic interventions for gastric variceal bleeding.








