Summary
The present network meta-analysis showed that the remote ischemic conditioning (RIC) was correlated with significantly lower infarction size compared to that in the ischemic postconditioning (IPC). No significant superiority of the RIC group vs. IPC group was discovered in this meta-analysis relating to complete ST-segment resolution incidence, mortality, re-infarction or target vessel revascularization. Further well-designed clinical trials should be conducted in the future in an attempt to provide better clinical outcomes for patients.
Introduction
Due to the higher morbidity and the enhanced mortality [1], ST-segment elevation myocardial infarction (STEMI) causes numerous public health problems and carries remarkable societal burden. Primary percutaneous coronary intervention (pPCI) for myocardial infarction and reperfusion in STEMI patients is the most effective method used to limit the size of myocardial infarction (MI). A smaller myocardial infarction size preserves the function of the left ventricular systolic function and reduces the impact on heart failure [2].
However, the process of opening infarct-related arteries can induce ischemia reperfusion injury (IRI) [3]. This phenomenon can paradoxically decrease the beneficial effects of PCI. Clinically, appropriately 50% of the STEMI myocardial injury is due to reperfusion [3]. How it might be possible to reverse this phenomenon is an issue that researchers strive to identify solutions for, some of which include various mechanical approaches and pharmacological methods [4, 5]. The strongest intervention for animals with reperfusion is remote ischemic conditioning (RIC), which can be applied before and during sustained ischemia and oneset of reperfusion [6, 7]. Moreover, ischemic postconditioning (IPC) has also demonstrated capabilities to reduce the myocardial injury or damage.
To date, there is no direct evidence for comparing effects of RIC and IPC on myocardial ischemia or injury. However, there are many randomized controlled trials (RCTs) comparing the effects of RIC and normal pPCI (RIC vs. pPCI) or comparing the effect of IPC and normal pPCI (IPC vs. pPCI) on myocardial ischemia in STEMI patients. Therefore, the present study conducted a indirect comparison using a network meta-analysis (NMA) for comparing different effects on myocardial ischemia in STEMI patients.
Aim
Direct comparative studies of the clinical efficiency between RIC and IPC are insufficient. Whether the clinical efficiency between RIC and IPC differs is uncertain. Therefore, this study aimed to compare the cardioprotective effects between RIC and the IPC in patients demonstrating STEMI using the present meta-analysis.
Material and methods
Search strategy
This NMA meta-analysis was conducted based on PRISMA guidelines [8]. This study included the articles published in the following database: Cochrane Central Register of Controlled Trials databases, PubMed and EMBASE, all of which were updated until September 15th, 2018. Keywords were used as search terms: ischemia-reperfusion injury, ischemic postconditioning, remote ischemic conditioning, pPCI and STEMI. Citations were screened for the title and abstract level and retrieved if they met our inclusion criteria. The references cited in all of the above information were also screened and reviewed.
Eligibility criteria
The studies were considered to be eligible if they accorded with a few inclusion criteria, including: 1) RCTs that compared post conditioning with remote ischemic conditioning in STEMI patients who underwent the treatment of pPCI. 2) The full text should be available for the selected studies. 3) The studies involved at least one of the following: enzymatic myocardial infarction size evaluated with serum peak creatinine kinase (CK) and peak creatinine kinase MB (CK-MB), the AUC of the CK-MB, the magnetic resonance imaging (MRI) evaluated infarction size, troponin T, troponin I or the AUC of troponin T, electro-cardiographic complete ST-segment resolution (with the percentage more than 70% or more than 50%) (cSTR), target vessel revascularization (TVR), re-infarction and mortality of patients during periods of follow-up. cSTR was calculated as a percentage of the value obtained from the basal ECG. A reduction > 70% or > 50% of the initial value was considered significant. TVR was assigned as reperfusion of the coronary artery bypass graft or re-intervention of the coronary artery of patients during the observation process and period.
Trials were excluded if: 1) The sample of the research literature was included, and the available evaluation index data was included. 2) High probability of repeatedly published literature.
Data search and quality assessment
The former selected articles were checked by inviting at least two researchers to decide on inclusion for the present NMA meta-analysis. Any divergences which were identified were checked using adjudication or consensus by inviting another investigator. The extracted data were included as the following: authors’ first name, publication years or date, characteristics of the patients, and the evaluative clinical outcomes. The primary endpoint used for the present NMA meta-analysis was the size of the enzymatic myocardial infarction, which was evaluated by using the serum peak CK, peak CK-MB, AUC of CK-MB, and troponin T, troponin I or AUC of troponin T. The secondary endpoints were the size of the infarction assessed by MRI, cSTR, TVR, re-infarction and all-cause mortality. The risk of bias and methodological quality were evaluated in duplicate using the Cochrane Collaboration tool [9].
Statistical analysis
Data were analyzed using Stata software (version: 12.0, Stata Corporation, College Station, TX, USA). Meanwhile, Review Manager (version: 5.3) purchased from RevMan (The Nordic Cochrane Centre, Beijing, China) was also employed to analyze the data. The categorical variables used the odds ratio (OR) and a 95% CI as the efficacy analysis statistic. Continuous variables used the standard mean difference (SMD) and a 95% CI as the efficacy analysis statistic. The continuous variables were represented as the median ± standard deviation (SD). The reference-based indirect comparative approach (NMA analysis) for meta-analysis involves synthesizing the data deriving from the distinguished interventions. Meanwhile, the indirect evidence that compares both A with C, and B with C together is analyzed integratedly [10]. The adjusted indirect treatment comparison model was established to conduct data analysis. A funnel plot was drawn to check for publication bias [11]. A two-tailed p < 0.05 was considered as statistically significant. The authors are solely responsible for the design and implementation of this study and its final contents.
Results
Included studies
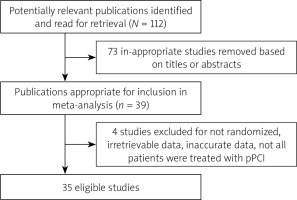
The numbers of studies identified at each stage of the meta-analysis are shown in Figure 1. Initially, the document search was processed and produced 112 promising literature articles. After reviewing literature titles and abstracts, a total of 73 documents were excluded. Therefore, 39 potential articles (full text) were retained [12–50]. After applying our predefined inclusion criteria, 4 studies were excluded due to: 1) not being randomized [12], 2) containing irretrievable data [13], 3) containing inaccurate data [14], or 4) not all pPCI having been conducted [15]. Finally, a total of 35 articles were included in this systematic meta-analysis [16–50].
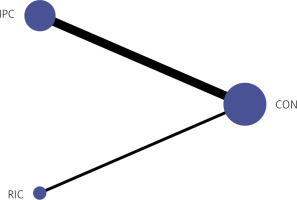
Among the 35 articles, total of 1941 patients underwent IPC treatment and a total of 404 patients underwent RIC treatment. Figure 2 shows the network plot of pair-wise comparisons from the included trials among the IPC group, RIC group and CON group.
Studies included in the assessments
A total of 27 studies involved patients administered IPC and/or RIC and individual persons (CON group), including 11 studies measuring troponin levels [20, 26, 28, 33, 34, 36, 40, 42, 44, 46, 48] and 16 studies measuring CK or CK-MB [16, 18, 19, 22–24, 27, 31, 32, 37, 39, 43, 45, 47, 49, 50] (Table I). Moreover, 15 studies measured the CMR [17, 19, 21, 22, 24–26, 29, 30, 35, 38, 42, 44, 46, 48] and 11 studies measured the ST-segment resolution (STR) [16, 19, 20, 23, 28, 33, 35, 44, 47, 49, 50] (Table I).
Table I
Demographic and clinical characteristic of included trials
Risk for bias
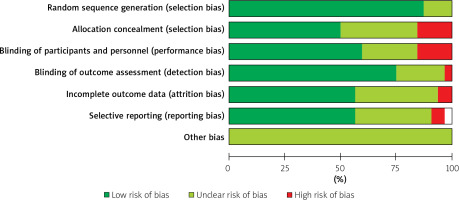
All the eligible trials were published between 2005 and 2018. The studies by Lonborg et al. [34, 35], Laskey et al. [31, 32] and Hahn et al. [28, 29] were reported in two publications each; therefore, we included the data as one study for each group. The risk for bias was assigned as the percentages (%) across all of the included studies. The analysis of study quality in the 35 eligible studies is presented in Figure 3. There was no high risk of bias for the random sequence generation (as selection bias), while high risk of bias mainly appeared for the allocation concealment (as selection bias) and blinding of participants and personnel (as performance bias) (Figure 3). However, only lower percentages of high risk of bias appeared for blinding of outcome assessment (as detection bias), incomplete outcome data (as attrition bias) and selective reporting (as reporting bias) (Figure 3). Moreover, all the other biases were demonstrated as the unclear risk of bias (Figure 3). Therefore, allocation concealment (as selection bias) and blinding of participants and personnel (as performance bias) were at high risk of bias (Figure 3), which should be paid attention to clinically.
Study characteristics
The baseline characteristics of included studies are described in Table I. Among them, 25 of the studies contained data (or results) on the peak or AUC of biomarkers in myocardial injury. Electrocardiographic ST-segment resolution was measured in ten studies. Fourteen studies illustrated the data for infarction size with the cardiac magnetic resonance (CMR) values. Out of the included studies, the patients’ mean age was 59.86 years, and the mean percentage of male is 77.22% (Table I). The mean percentage of diabetes mellitus in 33 studies was 18.81% (3.38% to 41.67% Table I). The mean percentage of dyslipidaemia in 28 studies was 48.53% (6.45% to 76.67% Table I). The mean percentage of hypertension in 33 studies was 46.58% (22.34% to 84.1%, Table I). Meanwhile, some patients were also administered β-blocker in 6 studies (6.94% to 98.41%), statins in 12 studies (8.33% to 100%) and angiotensin converting enzyme inhibitor (ACE-I)/agiotensin II receptor blockers (ARB) in 6 studies (9.21% to 96.98%) (Table I). Moreover, for treating the patients at the time of PCI, aspirin (in 24 studies), clopidogrel (in 24 studies), heparin (in 19 studies) and glycoprotein II b/III a inhibitor (in 7 studies) were the most common applied drugs (data not shown). Meanwhile, nitroglycerine (in 1 study), bivalirudin (in 2 studies), enoxaparin (in 1 study), abciximab (in 1 study), antiaggregants (in 1 study) and ticagrelor (in 1 study) were also administered for treating at the time of PIC, especially for the strategy of aspirin plus heparin plus clopidogrel, which was administered in 11 studies, and the strategy of heparin plus aspirin plus clopidogrel plus glycoprotein II b/III a inhibitor, which was administered in 6 studies (data not shown).
Infarction size was estimated by biomarkers of myocardial injury
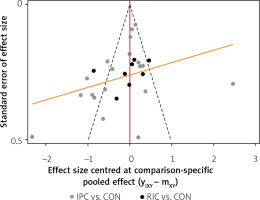
A total of 27 trials included 2,793 subjects (IPC: 1191, RIC: 278, CON: 1,464) in an indirect comparison analysis. Of the 27 studies, troponin levels were measured in eleven [20, 26, 28, 33, 34, 36, 40, 42, 44, 46, 48], sixteen studies contained data relating to the peak, or AUC of the CK or CK-MB [16, 18, 19, 22–24, 27, 31, 32, 37, 39, 43, 45, 47, 49, 50]. Figure 4 illustrates the comparison adjusted funnel plot. Funnel-plot images for cardiac biomarkers or molecules did not demonstrate obvious asymmetry, and it could be assumed that the study was less affected by publication bias.
Infarction size as estimated by cardiac biomarkers
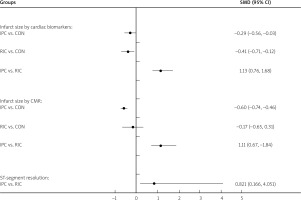
Pooled results showed that lower enzymatic infarction size was associated with the RIC group using the cardiac biomarkers in comparison with infarction size of the IPC group (IPC vs. RIC: SMD = 1.13; 95% CI: 0.76–1.68) (Figure 5). Moreover, according to infarct size by cardiac biomarkers, SMD value for IPC compared with CON, RIC compared with CON, IPC compared with RIC was –0.29 (95% CI: –0.56, –0.03), –0.41 (95% CI: –0.71, –0.12) and 1.13 (95% CI: 0.76–1.68), respectively (Figure 5).
Infarction size as estimated by cardiac magnetic resonance
In the included studies, CMR was measured in fifteen studies [17, 19, 21, 22, 24–26, 29, 30, 35, 38, 42, 44, 46, 48]. As shown in Figure 5, RIC remarkably decreased the size of infarction, assessed using CMR compared with IPC (IPC vs. RIC; SMD = 1.11; 95% CI: 0.67–1.84). Furthermore, based on infarct size by CMR, the SMD value for IPC versus CON, RIC versus CON, IPC versus RIC was –0.60 (95% CI : –0.74, –0.46), –0.17 (95% CI: –0.65, 0.31) and 1.11 (95% CI: 0.67–1.84), respectively (Figure 5).
Myocardial reperfusion injury evaluation using ST-segment resolution
The data for complete ST-segment resolution were obtained from all of the 11 studies. Among them, 70% of STR was considered to have completely declined in 7 studies and 50% in four studies. The results revealed a trend to greater cSTR values in RIC patients compared with IPC patients (OR = 0.821; 95% CI: 0.166–4.051) (Figure 5).
Clinical outcomes
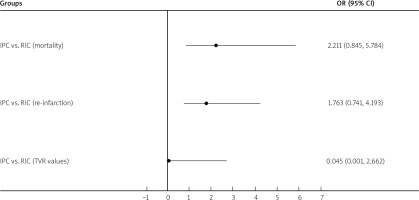
Ten studies reported re-infarction events [16, 19, 22, 23, 25, 29, 33, 35, 36, 41], eight studies reported TVR events [16, 19, 22, 23, 25, 35, 36, 44], and fourteen studies reported all-cause mortality events [16, 19, 20, 22, 23, 25–27, 29, 33, 35, 41, 42, 44]. There were no significant differences for mortality (OR = 2.211; 95% CI: 0.845–5.784), re-infarction (OR = 1.763; 95% CI: 0.741–4.193) or TVR values (OR = 0.045; 95% CI: 0.001–2.662) between RIC patients and IPC patients (Figure 6).
Discussion
The present NMA meta-analysis included the data deriving from 35 randomized studies including a total of 4692 STEMI patients who underwent primary PCI. The results revealed that RIC was related to a significantly lower infarct size compared to that in the IPC. However, in this meta-analysis regarding the cSTR incidence, IPC showed better protection than RIC. No significant superiority of RIC vs. IPC was discovered in all-cause mortality, re-infarction or TVR.
Final infarction size was significantly related to the occurrence of myocardial reperfusion injury. Thus, weakening myocardial reperfusion injury with the appropriate therapeutic strategies or methods is critical for determining the infarction size. The protective role of RIC and IPC against reperfusion injury was introduced in the laboratory test or experiments and the clinical trials. However, no head-to-head trial between IPC and RIC has been reported so far. In the rat model of MI [51], we observed a trend toward smaller infarct size in RIC groups compared with IPC subjects. Eitel et al. [22] found that combining RIC with IPC remarkably enhances the myocardial salvage compared to that in the conventional pPCI. However, IPC alone failed to act a cardio-protective effect on STEMI patients treated with pPCI. Our analysis showed a significant reduction in infarct size with RIC compared to the IPC group using either biomarker release or CMR. The results might be associated with many factors, including: 1) IPC was performed by repetitive interruption of coronary blood flow at the site of the culprit coronary lesions. This protocol may induce coronary micro-embolization and further myocardial injury, which can reduce the beneficial effect of IPC [52]. 2) Manual thrombus aspiration (MTA) as an adjunct was performed in several studies included. However, in the original proof-of-concept studies of Stata, postconditioning was applied immediately after reperfusion. According to animal studies [53], the time since the withdrawal of the thrombectomy device, after the MTA procedure, results in loss of cardio-protection. By being performed at the upper or lower limbs away from the heart, RIC can avoid the aforementioned shortcomings.
cSTR is considered to be a surrogate of efficient microvascular reperfusion and correlates well with the 1-year mortality in patients with STEMI [54]. Our meta-analysis failed to reveal significant changes or improvement for cSTR rate in the RIC group compared to that in the IPC group. However, there were trends toward more cSTR rate in RIC group. Of the 3 RIC studies included, one included patients only with anterior infarctions. Subgroup analyses in other studies suggested cSTR was more frequently observed in patients with anterior versus non-anterior myocardial infarction. An anterior infarction is generally associated with a high-risk STEMI with a large myocardium area at risk; therefore, these results might support active application of the RIC for patients with high risk during the primary PCI.
Our study did not reveal differences in the hard clinical endpoints between RIC group and IPC group. Although enzymatic and CMR-derived infarction size is a potent surrogate marker of future cardiovascular events, a large sample size of patients and a longer follow-up time [55] are required to determine whether the RIC protocol can translate into clinical outcomes. However, the inadequate sample size and different follow-up lengths among different studies included had little impact on the clinical outcome. Moreover, we also agreed that the outcomes at follow-up might have interfered with confounding factors such as compliance with medication, diet and lifestyle modification measures. Therefore, more studies are needed as confirmatory evidence of clinical outcomes.
Several potential limitations should be noted: Firstly, some of the heterogeneity across investigations (such as use of thrombectomy and GPIIb/IIIa inhibitors, application of the RIC/IPC protocol) might affect the interpretation and the generalization of the present meta-analysis findings. Secondly, RCTs comparing RIC with controls that were included were dependent on the relatively small sample size and the limited number of patients. Further well-designed multicenter investigations (such as CONDI2: NCT01857414 and ERIC-PPCI: NCT02342522) are also required. Thirdly, the effects of RIC on myocardial ischemia might not be specific to the STEMI patients, and might play a positive role in the other non-STEMI patients [56]. However, this study only investigated effects of RIC or PCI on myocardial ischemia in STEMI patients, and not on other diseases causing myocardial ischemia.
In summary, RIC would be a potential adjunctive therapeutic strategy to PCI for protecting against reperfusion injury in STEMI patients. Furthermore, we also anticipated that the present research would establish efficacy of RIC to protect against cardiac disorders, cerebrovascular events and other injuries clinically. RIC demonstrates promising neuroprotective effects on patients with a risk of cardiovascular diseases, especially for the risk of IR injury.
Conclusions
The present NMA meta-analysis showed that RIC was correlated with significantly lower infarction size compared to that in IPC. No significant superiority of the RIC group versus the IPC group was found in this meta-analysis relating to cSTR incidence, mortality, re-infarction and TVR. Further well-designed clinical trials should be conducted in the future in an attempt to provide better clinical outcomes for patients.