Introduction
In patients with decompensated cirrhosis, despite increased cardiac output, there is a state of systemic hypovolemia with splanchnic pooling of blood and decreased peripheral vascular resistance, resulting in reduced renal perfusion with hepatorenal syndrome (HRS). In addition, hypovolemia, diuretics, infections, etc. can precipitate acute kidney injury (AKI) in these patients. Fagundes et al. found that the modified AKI classification, which redefined AKI as serum creatinine (sCr) values (0.3 mg/dl or 50% increase from baseline) improved the risk stratification of patients with AKI [1]. It was further adopted in 2015 by the International Club of Ascites (ICA), resulting in earlier detection of AKI at lower sCr values, earlier treatment initiation, and better outcomes [2]. The CANONIC study revolutionized hepatology practice in 2013 by classifying patients admitted with acute decompensation as having acute-on-chronic liver failure (ACLF) based on organ failures [3]. They included sCr >2 mg/dl as renal failure and patients with sCr 1.5-2 mg/dl as ACLF grade 1 if associated with one non-renal organ failure, considering their increased mortality as compared to non-ACLF patients. The ICA-AKI criteria were determined in decompensated cirrhosis.
We know that ACLF grading is based on organ failures, which increase short-term mortality. The renal dysfunction in ACLF is multifactorial with pre-renal (hypovolemia due to upper gastrointestinal bleeding, diarrhea, etc.), sepsis, circulatory failure, drugs such as diuretics and antibiotics, hyperbilirubinemia, cholemic nephropathy, etc. being responsible. In addition, due to reduced muscle mass, reduced creatine synthesis, and assay interference by high bilirubin, sCr is underestimated in ACLF patients. Terlipressin is a splanchnic and systemic vasoconstrictor that reduces splanchnic pooling of blood [4, 5]. In addition to reducing portal pressure, it also reduces bacterial translocation and the resultant cytokine storm. Terlipressin was found to be effective in the reversal of HRS in patients with HRS type 1 in the presence of SIRS in another study [6]. Arora et al. showed terlipressin to be better than noradrenaline in ACLF patients with HRS-AKI [7]. Terlipressin has a role in microcirculation and therefore may act better in continuous infusion as compared to bolus especially in the presence of sepsis and circulatory failure. Additionally, there is a difference in the dose requirement and subsequent side effects. The continuous infusion dose is 2 mg/24 hours, to be increased by 1 mg/day and bolus doses start from 1 mg every 6 hour up to a maximum of 12 mg/day. Terlipressin has inherent adverse drug reactions (ADR) as ischemic complications especially peripheral ischemia (digital gangrene), mesenteric ischemia (diarrhea, melena), myocardial ischemia (chest pain, dyspnea), etc., requiring drug discontinuation. Due to major differences in total daily dose we hypothesized that the infusion group should have better efficacy with less ADR. Therefore, we compared intravenous continuous infusion vs. bolus doses of terlipressin in patients with ACLF-AKI defined as per the 2015 ICA-AKI criteria.
Material and methods
It was a prospective, open-label, parallel randomized controlled study, conducted at a tertiary care center, in the Hepatology Clinic and Department of Medicine, from January 2019 to June 2020. The institutional ethics committee (IEC) approved the study (approval no. BREC/Th/18/Med/02 dated February 28, 2019). The study was conducted in accordance with the Declaration of Helsinki’s latest amendment in 2013. All study participants gave written informed consent prior to study enrollment.
Patients
All patients admitted with ACLF as defined by the CANONIC study were screened for AKI using the 2015 ICA-AKI criteria. AKI was defined as a rise in sCr ≥ 0.3 mg/dl (≥ 26.5 µmol/l) or ≥ 50% of the baseline value within 48 hours [3]. The sCr value from the last 7 days (if available) or any value from the last 3 months was taken as the baseline value. AKI staging was done as per 2015 ICA-AKI criteria [2]. All patients with ACLF-AKI were included in the study. The following patients were excluded from the study: age < 18 or > 70 years, pregnancy, history of cardiovascular disease, cerebrovascular disease, chronic obstructive airway disease, chronic renal disease, hepatocellular carcinoma, and other malignancies. All patients with ACLF-AKI received volume expansion with crystalloids and, if required, intravenous albumin at 1 g/kg/day for 48 hours or a maximum of 100 g/day. Terlipressin was started if sCr did not decrease by 25% of its baseline value. Patients were allocated in a 1 : 1 ratio to the terlipressin continuous intravenous infusion (Terli-I) and bolus (Terli-B) groups. Eligible participants were randomly assigned by computer generated random numbers to one of the two intervention groups. TG did a random allocation sequence; AG enrolled participants and assigned interventions to participants. In the Terlipressin Infusion (Terli-I) group, continuous IV infusion was started at 2 mg/24 hours, and in the Terlipressin Bolus (Terli-B) group, it was started at 1 mg/q6h. If sCr did not decrease by 25% of the baseline value, the terlipressin infusion and bolus were both increased by 1 mg every 48 hours, up to a maximum dose of 12 mg/day.
Primary end points
Response to treatment was assessed by regression, progression, or stable AKI. AKI regression was defined as improvement in the AKI stage moving to a lower stage with a full or partial response. The final sCr value within 0.3 mg/dl of the baseline sCr value was defined as a full response, and if it remained ≥ 0.3 mg/dl above the baseline sCr value, it was defined as a partial response. The progression of AKI was defined as the AKI stage worsening to a higher stage. Stable AKI was defined as no regression in the stage of AKI with treatment. Progression of AKI and stable AKI were considered as non-response of AKI to treatment.
Secondary end points
After treatment, 28- and 90-day mortality were taken as secondary end points. Secondary end points included the cumulative doses of terlipressin required for a response to treatment and the length of hospital stay.
Responders and non-responders
Responders in this study were defined as all patients who had a full or partial response and a final sCr of 1.5 mg/dl. Non-responders were defined as all patients with final sCr > 1.5 mg/dl.
Statistical analysis
The primary end point of a complete response was taken into consideration for the estimation of the sample size of the study. A previous study found that the complete response to terlipressin continuous infusion was 55% and the complete response to terlipressin bolus doses was 46% [8]. Assuming a 95% confidence interval, α error of 5% and β error of 10%, a sample size of 48 in each group was calculated. All continuous variables were presented as mean ±SD (range) or median [IQR; Q1, Q3], while categorical variables were presented as frequency and percentages. The Mann-Whitney U test or Student t-test was used to compare continuous variables, whereas the chi-square and Fisher exact tests were employed to compare categorical variables. Survival analysis was used to determine mortality at 28 and 90 days. Per-protocol analysis was done in this study. P < 0.05 was considered significant. For the analysis, IBM’s SPSS v21.0 (IBM, USA) was employed.
Results
Patients
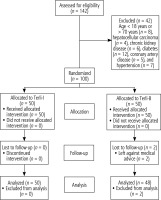
A total of 142 patients having ACLF with AKI were assessed for eligibility. Forty-two patients were excluded from the study because they were 18 or older (n = 8), had hepatocellular carcinoma (n = 4), chronic kidney disease (n = 6), diabetes mellitus (n = 12), coronary artery disease (n = 5), or had hypertension (n = 7). One hundred patients were randomly assigned to the Terli-I (n = 50) and Terli-B (n = 50) groups. In the Terli-B group, two patients left against medical advice during hospitalization. Finally, 50 patients in the Terli-I group and 48 patients in the Terli-B group were analyzed (Fig. 1). All baseline characteristics were comparable between the Terli-I (n = 50) and Terli-B (n = 48) groups, with the exception of Terli-I, having a longer prothrombin time (PT) (25.9 vs. 21.8, p = 0.04), higher international normalized ratio (INR) (2.05 vs. 1.7, p = 0.02), higher Child-Turcotte Pugh score (CTP) (12.1 vs. 10.4, p ≤ 0.001), and higher Model for End stage Liver Disease (MELD) score (30.2 vs. 27, p = 0.03) than the Terli-B group, respectively (Table 1).
Table 1
Baseline characteristics
| Terli-I (n = 50) Mean ±SD or median [IQR; Q1, Q3] | Terli-B (n = 48) Mean ±SD or median [IQR; Q1, Q3] | P-value | |
|---|---|---|---|
| Age (years) | 44.0 ±8.5 | 45.04 ±9.4 | 0.565 |
| Sex (male : female), n | 50 : 0 | 47 : 1 | – |
| Etiology of cirrhosis, n ALD/HBV/HCV/NASH | 45/3/2/0 | 46/0/1/1 | NS |
| ACLF grade 1/2/3 | 22/9/19 | 24/17/7 | NS |
| Acute event§, n Sepsis/AH/UGIB/AVH | 41/25/4/2 | 37/22/8/1 | NS |
| AKI stage 1/2/3 | 3/21/26 | 16/17/15 | NS |
| Baseline MAP (mmHg) | 69 ±8 | 74 ±11 | 0.07 |
| TLC (× 103/mm3) | 15.5 [9.15, 19.0] | 11.6 [6.2, 19.2] | 0.084 |
| Platelets (× 109/l) | 100.0 [70.0, 150.0] | 120.0 [82.5, 170.0] | 0.121 |
| Bilirubin (mg/dl) | 5.65 [2.3, 14.9] | 5.3 [2.07, 16.1] | 0.865 |
| PT | 25.9 [19.9, 29.1] | 21.8 [17.1, 29.3] | 0.049 |
| INR | 2.05 [1.6, 2.6] | 1.70 [1.4, 2.4] | 0.023 |
| Albumin (gm/dl) | 2.38 ±0.33 | 2.50 ±0.46 | 0.144 |
| Serum creatinine (mg/dl) | 2.4 [1.8, 3.2] | 2.1 [1.8, 3.02] | 0.298 |
| CTP | 12.10 ±1.81 | 10.45 ±2.08 | < 0.001 |
| MELD | 30.21 ±6.87 | 27.08 ±6.57 | 0.033 |
§ Some patients had more than one acute event. ALD – alcoholic liver disease, AH – alcoholic hepatitis, AVH – acute viral hepatitis, AKI – acute kidney injury, CTP – Child-Turcotte Pugh score, HBV – hepatitis B, HCV – hepatitis C, INR – international normalized ratio, MELD – Model for End stage Liver Disease, NASH – non-alcoholic steatohepatitis, MAP – mean arterial pressure, PT – prothrombin time, Terli-I – Terlipressin infusion, Terli-B – terlipressin bolus, TLC – total leukocyte count, UGIB – upper gastrointestinal bleed
Distribution of ACLF and AKI
The distribution of ACLF was grade 1 (22 vs. 24), grade 2 (9 vs. 17), and grade 3 (19 vs. 7) in Terli-I and Terli-B groups, respectively. The distribution of AKI was stage 1 (3 vs. 16), stage 2 (21 vs. 17), and stage 3 (26 vs. 15) in Terli-I and Terli-B groups, respectively. The most common acute event in ACLF was sepsis in 41 and 37 patients in the Terli-I and Terli-B groups, respectively (Table 1). The distribution of sepsis was spontaneous bacterial peritonitis (SBP)/pneumonia/cellulitis/urinary tract infections (UTI)/unknown in 21/4/4/1/11 and 18/9/1/2/7 patients in Terli-I and Terli-B groups, respectively.
Primary end points
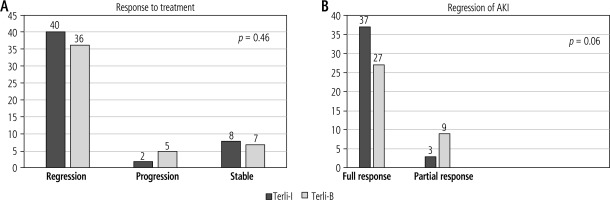
The regression of AKI was present in 40 vs. 36 patients in the Terli-I (n = 50) and Terli-B (n = 48) groups, respectively. The progression of AKI was present in 2 vs. 5 patients in the Terli-I and Terli-B groups, respectively. AKI was stable in 8 of the Terli-I and 7 of the Terli-B groups, respectively. In subgroup analysis of regression of AKI, full and partial responses were present in a higher number of patients in the Terli-I group than the Terli-B group (37 vs. 27 and 3 vs. 9, p = 0.06), respectively (Fig. 2).
Secondary end points
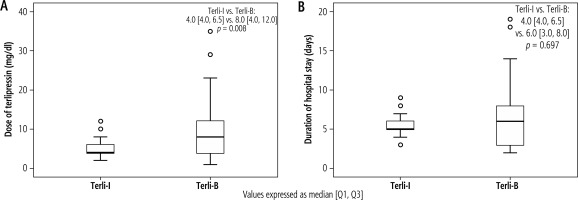
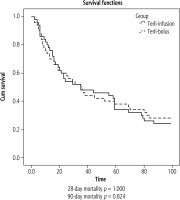
The mortality rates at 28 and 90 days were not different between the Terli-I and Terli-B groups (23 vs. 23, p = 1.0, and 15 vs. 13, p = 0.65) (Fig. 3). The mean cumulative dose of terlipressin in the Terli-I group was significantly lower than in the Terli-B group (4.0 vs. 8.0 mg/dl, p = 0.008). The mean duration of hospital stay was less in Terli-I than in Terli-B (4 vs. 6 days, p = 0.697, respectively) (Fig. 4). Interestingly, no patient in Terli-I had any adverse events. In contrast, in Terli-B, 15 out of 48 patients had some adverse events: diarrhea in 9, abdominal pain in 4, dyspnea in 1, and myocardial ischemia in 1 patient. All adverse events were reversible with discontinuation of terlipressin (Table 2).
Fig. 3
Kaplan-Meier analysis for 28- and 90-day mortality between Terli-I (n = 50) and Terli-B (n = 48) groups
Responders vs. non-responders
Out of a total of 98 patients, 71 were responders and 27 were non-responders. Among the 71 responders, Terli-I had 38 patients and Terli-B had 33 patients. Among non-responders, Terli-I had 12 and Terli-B had 15 patients. Responders had a lower MELD score as compared to non-responders. There was no significant difference in duration of hospital stay or cumulative dose of terlipressin between the two groups (Table 3). Non-responders had significantly higher grades of ACLF with grade 3 in 12 (44%), rade 2 in 8 (30%), and grade 1 in 7 (26%), as compared to responders who had ACLF grade 3 in 14 (20%), grade 2 in 18 (25%), and grade 1 in 39 (55%), respectively, with p < 0.0001 (Fig. 5A).
Fig. 5
Comparison of ACLF grades (A) between responders (n = 71) and non-responders (n = 27). The data are presented as percentages. B) Kaplan-Meier analysis of responders (n = 71) and non-responders (n = 27)
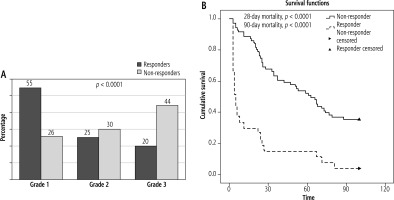
Table 3
Comparison of responders (n = 71) and non-responders (n = 27)
Discussion
Our study illustrates that terlipressin administration by continuous versus bolus doses has no statistically significant difference in the regression or progression of AKI; however, the Terli-I group had a full response (sCr decreased to a value within 0.3 mg/dl of the baseline value) in a numerically higher number of patients as compared to the Terli-B group. As compared to the bolus group, Terli-I is better with regard to its safety profile: no adverse events and a low cumulative dose requirement. Interestingly, non-responders (final sCr > 1.5 mg/dl) had higher grades of ACLF as compared to responders (final sCr < 1.5 mg/dl).
The hemodynamic effect of an intravenous bolus of terlipressin lasts for 4-6 hours and needs to be repeated in 4-6 hours. On the other hand, continuous infusion has a persistent hemodynamic effect, which is desirable in this patient population. Terlipressin is a systemic vasoconstrictor as well and is known to cause ischemic complications. The present study showed adverse events in 15 patients, which included abdominal pain and diarrhea in 5 and 9 patients, respectively, which are likely related to mesenteric ischemia. One patient had dyspnea, and another had myocardial ischemia. All these adverse events were reported in the terlipressin bolus group, requiring drug discontinuation, and no ADR was reported in the infusion group. This is in contrast to the study by Cavallin et al., where they reported severe ADR in 20% of the terlipressin infusion group as compared to 43% in the bolus group [8]. On the other hand, the CONFIRM study investigators observed adverse events including abdominal pain, diarrhea, and respiratory failure in 14% of patients in the terlipressin group as compared to 5% in the placebo group [9]. Both these studies were performed in decompensated cirrhosis patients. The adverse effects of terlipressin are related to its vasoconstrictive effects. The infusion leads to continuous administration of the drug over 24 hours with steady plasma levels. Antonio et al. suggested that intermittent bolus doses of terlipressin cause rebound of effects and a non-stable clinical situation [10]. Though in the present study, we did not measure pharmacological levels of terlipressin, it is likely that the higher peak dose effect in the bolus group led to adverse effects related to mesenteric and peripheral ischemia.
The introduction of the concept of ACLF is a milestone in understanding the pathophysiology of acute decompensation events in cirrhosis. In the CANONIC study, the investigators found mean serum creatinine values higher in the subgroup of patients with single non-renal organ failure who had 28-day mortality as compared to survivors (1.3 vs. 0.9 mg/dl, p < 0.0001) [3]. This was a key observation, as identifying these patients when they qualify for grade 1 ACLF (sCr 1.5-1.9 mg/dl) or renal failure (> 2 mg/dl) would be too late for interventions. Considering this, we decided to include patients with ACLF with AKI as defined per the ICA-AKI criteria. It was evident from the present study that it was the ACLF grade that was a determinant of response to therapy. The non-responders group had almost 74% of patients in ACLF grades 2 and 3. Piano et al. also reported decreasing response to HRS resolution in patients with higher grades of ACLF, with 60% in ACLF grade 1, 48% in ACLF grade 2, and 29% in ACLF grade 3 with terlipressin and albumin therapy [10]. In addition to renal dysfunction, the presence of other organ failures becomes crucial for the final outcome of these patients. Studies have suggested that clinical course of ACLF correlates with levels of systemic inflammatory markers instead of circulatory functions alone [11]. The increased cytokine levels reduce vital organ perfusion by causing left ventricular dysfunction, vasodilation, microcirculatory dysfunction, and finally apoptosis. Studies from all over the world have shown sepsis to be the most common acute event in ACLF [12–14]. Gomez et al. described the renal adaptive response in sepsis induced AKI [15]. Sepsis induces microcirculatory disturbances with focal attenuation of tubular and glomerular capillaries, resulting in heterogenous foci of hypoxia and decreased perfusion in the kidneys. Additionally, increased luminal levels of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) induce a tubular epithelial cell adaptive response with metabolic downregulation leading to reduced energy expenditure, shifting to a phase with cell cycle arrest to reduce DNA damage and shifting the mitochondrial ATP usage favoring cell survival mechanisms which culminates in sacrifice of the tubular absorptive and secretory function, transiently to overcome the period of crisis [16, 17]. Due to reduced absorptive function, increased delivery of sodium and chloride load to the macula densa in distal tubules leads to tubuloglomerular feedback (TGF), reducing glomerular filtration in early sepsis [18, 19]. If the systemic inflammatory response is not adequately addressed in the early stages, tubular injury will occur, leading to irreversible damage. As a result, an aggressive approach that includes volume expansion, vasoconstrictors, and treatment aimed at the cause and removal of precipitating factors for AKI may improve the overall outcome of these patients.
Our study has a few limitations. First, the number of patients was low, so subgroup analysis for different grades of ACLF could not be done. Second, inflammatory and urinary biomarkers were not studied because the study was not designed. Third, patients in the terlipressin bolus group who required drug discontinuation due to ADR were not started on continuous infusion, as this was not originally designed in the study. In future, large, double blind, randomized controlled trials of vasoconstrictors in ACLF are needed to confirm these findings, and considering the heterogeneity of ACLF-AKI pathogenesis, new therapeutic strategies should be investigated for treatment as well as prevention of AKI in ACLF patients.
Conclusions
In conclusion, terlipressin is a key drug in the management of AKI. The ACLF-AKI is a condition with high short-term mortality, and therefore, terlipressin infusion should be a preferred modality as compared to bolus doses as it clearly reduces the cumulative dose and duration of hospital stay with no adverse events being reported. The beneficial effects of terlipressin, in addition to improvement in hemodynamics, may also include a reduction in systemic inflammation. However, the results may not be generalizable due to the small number of patients in this study and different etiologies for acute and chronic hepatic insults in different geographical areas.