Introduction
According to the global cancer statistics 2020, new lung cancer cases and deaths were estimated at 2.2 million and 1.8 million, respectively. In 2020, lung cancer ranked second in incidence and first in mortality; the incidence and mortality of lung cancer are the highest among those of all tumours in men, and third and second highest in women, respectively [1].
Lung cancer has gained increasing attention due to the high incidence and mortality. Low-dose computed tomography (LDCT) scan is an important means of lung cancer screening [2]. Indeed, the detection rate of small pulmonary nodules has gradually increased with the widespread use of lung LDCT screening, while prevention, early detection, and early treatment remain key focuses. In a Japanese study in which participants were followed for an average of 5.7 years, the 5-year survival rate of primary lung cancer detected by LDCT was 90% after surgical resection [3, 4]. In a Dutch randomized trial, volume lung computed tomography (CT) screening showed that the mortality of lung cancer in the CT lung screening group was significantly lower than that in the non-screening group among high-risk populations [5].
According to the guidelines of the American College of Chest Physicians (ACCP) for the evaluation and treatment of these lesions, pulmonary nodules can be treated with surgical resection in the following cases: 1) solid nodules with definite malignant signs or enlargement during follow-up; 2) solid component of the ground glass nodule identified during follow-up; and 3) part-solid nodules > 8 mm and persisting during follow-up, or the diameter of part-solid nodules > 15 mm at the time of initial detection [6].
The National Comprehensive Cancer Network (NCCN) guidelines highlight that surgery is the only treatment for most stage I non-small cell lung cancers. Regarding surgical methods, video-assisted thoracoscopic surgery (VATS) is minimally invasive compared to open surgery [7, 8]. VATS is the preferred and most commonly used surgical resection method for pulmonary nodule resection in clinical practice [9]. Importantly, VATS can accurately localize pulmonary nodules. However, some pulmonary nodules are small, far from the pleura, or they are ground-glass and subsolid nodules, in which case it is often difficult for the surgeon to determine the location of the pulmonary nodules during surgery and requires preoperative assisted localization [10]. Various positioning techniques are currently available in clinical practice.
Aim
This study aimed to discuss the application value and safety of 2 kinds of localization needles commonly used in clinical practice for pulmonary nodules (breast localization needles and anchor localization needles) under CT guidance before thoracoscopic surgery.
Material and methods
Patient population
A total of 216 patients with pulmonary nodules who underwent CT-guided percutaneous location at the First Hospital of Xiamen University between September 2020 and April 2022 were retrospectively enrolled. One of the patients developed pneumothorax during the localization process and the pulmonary nodules were not clearly displayed; therefore, pulmonary nodule localization was stopped. Finally, 215 patients with 247 pulmonary nodules were counted. This study was approved by the Ethics Committee of the First Hospital of Xiamen University (number: 2023028). All patients provided written informed consent before pulmonary nodule localization.
The criteria for preoperative assisted localization were decided according to the expert consensus workshop report and included the following: 1) single or multiple pulmonary nodules (< 1.5 cm in diameter or > 1.0 cm from the pleura); 2) the imaging manifestations were pure ground glass or subsolid nodules; and 3) it was difficult to locate the nodule by palpation intraoperatively. Preoperative assisted localization can be used if one or more of these criteria are met [10].
CT-guided pulmonary nodule localization
CT-guided pulmonary nodule localization was executed by interventional radiologists with more than 10 years of experience.
Based on the location of the pulmonary nodule, the patient was positioned appropriately during the CT scan (Siemens, Munich, Germany).
The location of the pulmonary nodule was shown on the CT image; we evaluated the feasibility and safety of the localization procedure and determined the most appropriate needle positioning path to avoid important structures such as blood vessels. In aseptic conditions, local anaesthesia (lidocaine) was injected; location was performed under CT guidance. We used 20 G breast localization needles (Argon Medical Devices, Plano, Texas, USA) or anchor localization needles (Senscure Biotechnology, NingBo, ZheJiang, China) (Photo 1). The time of the location and complications in the positioning process were recorded.
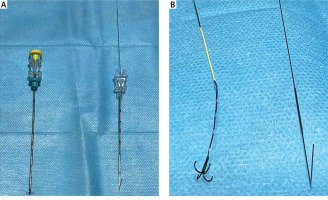
Photo 1
Comparison of the structure of the anchor localization needles (left) and 20 G breast localization needles (right); (A) overall structure of the anchor localization needles (left) and 20 G breast localization needles (right); (B) the structure after the positioning needle has been pushed out of the sheath. The anchor positioning needle consists of the 4-claw and tri-coloured positioning lines (left); 20 G breast localization needle consists of single hooks and a wire positioning needle (right)
Resection of pulmonary nodules
After successful general anaesthesia, the surgeon placed the patient in a contralateral decubitus position according to the left or right positions of the pulmonary nodules to be excised. Conventional disinfection was conducted after surgical drape. The midaxillary line or anterior axillary line on the same side of the pulmonary nodules was selected for incision, and then a thoracoscopic probe was placed to observe the pulmonary conditions. Finding the pulmonary nodules and the positioning needle, we lifted the lung tissue from the positioning needle and used an endovascular cutter to remove the pulmonary nodules, the positioning needle, and the lung tissue around the nodules, before subjecting these tissues to rapid freezing pathology. According to the pathological results, the scope of lung tissue resection and lymph node sampling were determined during the operation. Subsequently, intrathoracic haemostasis was performed and the lungs were checked for air leakage. Finally, the incision was sutured, and a thoracic drainage tube was fitted.
Data collection
The clinical data of patients were collected from their medical records, including age, sex, smoking history, emphysema, imaging morphology, pulmonary nodule location, localization numbers in each patient, pulmonary nodule size, the shortest distance between pulmonary nodules and the pleura or skin, localization time, type of localization needle, complications, detachment rate, visual analogue scale (VAS) score, surgical procedure, and surgical pathological results. The localization time was determined as the interval from the CT scan acquired before the localization to the one after the procedure was completed. Three types of imaging appearances were noted: ground-glass, part-solid, and solid. Pulmonary nodule locations are classified by both lung lobes and lung bands. The lung lobes are categorized as right upper, right middle, right lower, left upper, and left lower. The lung bands are categorized as periphery, middle, and inner. Pain was evaluated using a VAS, where 0 indicated no pain, 1–3 indicated mild pain, 4–6 indicated moderate pain, and 7–10 indicated severe pain. The surgical procedures encompassed pulmonary wedge resection, segmental resection, and lobectomy.
Statistical analysis
First, the normality of the measurement data was tested. Normally distributed data were statistically described by the average, and the t-test was used to compare differences between the groups, whereas non-normally distributed data were statistically described by the median, and the rank-sum test was used to compare differences between the groups. The χ2 test was used to compare the categorical variables. P-values < 0.05 were considered statistically significant. All analyses were performed with SPSS 28.0 (IBM, Armonk, NY, USA).
Results
Patient characteristics
As shown in Table I, a total of 247 pulmonary nodules were located in 215 patients, and 152 pulmonary nodules in 145 patients were diagnosed with lung adenocarcinoma by postoperative pathology. Regarding the patient characteristics for pulmonary nodule localization, the median age of localization patients and patients with lung adenocarcinoma was 48 years. Among the 215 patients who underwent localization, 145 (67.4%) were females and 102 of them (70.3%) had lung adenocarcinoma. None of the women had a history of smoking. The main morphological feature of localized pulmonary nodules was ground-glass nodule, accounting for 62.8% of cases. These nodules were mainly located in the right upper and right lower lobes. The median diameter of localized pulmonary nodules was 0.7 cm, 55.9% of the tumours had a diameter of 0.5–1.0 cm, and the location time was approximately 13 min. We used breast localization needles and anchor localization needles in 27.9% and 72.1% of cases, respectively. Complications included a small amount of pneumothorax in 50 (20.2%) patients and bleeding in the needle route in 127 (51.4%) patients. Only the size of the pulmonary nodules was statistically significant in the total localization pulmonary nodule and lung adenocarcinoma groups.
Table I
Patients’ characteristics
| Variables | All located patients (215 patients with 247 nodules) | All located patients with lung adenocarcinoma (145 patients with 152 nodules) | P-value |
|---|---|---|---|
| Age [years] | 48 (24–80) | 48 (26–80) | 0.655 |
| Female, n (%) | 145 (67.4) | 102 (70.3) | 0.422 |
| Smoking history (female/men) | 22 (0/22) | 12 (0/12) | 0.725 |
| Emphysema | 5 (2.3) | 3 (2.1) | 0.972 |
| Imaging morphology, n (%): | 0.206 | ||
| Ground-Glass | 155 (62.8) | 97 (63.8) | |
| Part-Solid | 30 (12.1) | 26 (17.1) | |
| Solid | 62 (25.1) | 29 (19.1) | |
| Location of pulmonary nodules, n (%): | 0.535 | ||
| Right upper | 83 (33.6) | 48 (31.6) | |
| Right middle | 19 (7.7) | 8 (5.2) | |
| Right lower | 61 (24.7) | 32 (24.7) | |
| Left upper | 55 (22.3) | 42 (21.1) | |
| Left lower | 29 (11.7) | 22 (14.5) | |
| Size of pulmonary nodules [cm]: | 0.045* | ||
| Median (range) | 0.7 (0.2–2.0) | 0.8 (0.6–2.0) | |
| ≤ 0.5, n (%) | 67 (27.1) | 24 (15.8) | |
| 0.5 >; ≤ 1.0, n (%) | 138 (55.9) | 91 (59.9) | |
| 1.0 >; ≤ 1.5, n (%) | 31 (12.6) | 28 ( 18.4) | |
| 1.5 >; ≤ 2.0, n (%) | 11 (4.5) | 9 (5.9) | |
| Depth to skin [cm]: | |||
| Median (range) | 5.9 (0.6–10.8) | 6.0 (0.6–10.8) | 0.617 |
| Depth to pleura [cm]: | |||
| Median (range) | 0.6 (0–4.5) | 0.6 (0–4.5) | 0.673 |
| Localization time [min]: | |||
| Median (range) | 13 (3–30) | 13 (3–27) | 0.815 |
| Localization needle, n (%): | |||
| Breast localization needles | 69 (27.9) | 48 (31.6) | 0.438 |
| Anchor localization needles | 178 (72.1) | 104 (68.4) | |
| Complications: | |||
| Pneumothorax | 50 (20.2) | 29 (19.1) | 0.777 |
| Alveolar haemorrhage | 127 (51.4) | 78 (51.3) | 0.984 |
Comparison of pulmonary nodule localization needles
The hookwire and anchor pulmonary nodule location needles are compared in Table II. We evaluated the differences between the 2 groups, including age, tumour size, depth to pleura, puncture location time, complications, detachment rate, and VAS. The differences between the 2 groups were mainly manifested in puncture location time, detachment rate, and VAS. The detachment rate (0%) and positioning time (median: 12 min) of the anchor group were less than those of the hookwire group (8.7% and median: 13 min, respectively). The median VAS was approximately 2 in the anchor group and 5 in the hookwire group.
Table II
Comparison of the 2 pulmonary nodule localization needles
| Variables | 20 G breast localization needles (62 patients with 69 nodules) | Anchor localization needles (153 patients with 178 nodules) | P-value |
|---|---|---|---|
| Age [years] | 50 (20–74) | 48 (26–80) | 0.845 |
| Tumour size [cm] | 0.7 (0.2–1.6) | 0.7 (0.2–2.0) | 0.495 |
| Depth to pleura [cm] | 0.5 (0–3.9) | 0.6 (0–4.5) | 0.106 |
| Puncture location time [min] | 13 (10–26) | 12 (3–30) | 0.001* |
| Alveolar haemorrhage | 29 (42.0) | 98 (55.1) | 0.066 |
| Pneumothorax | 9 (13.0) | 41 (23.0) | 0.080 |
| Detachment rate | 6 (8.7) | 0 (0) | < 0.001** |
| Visual analogue scale | 5 (4–6) | 2 (1–3) | < 0.001** |
We found no significant differences in age, tumour size, complications, or depth to pleura among the 2 groups. The proportion of alveolar haemorrhage and pneumothorax in the breast localization needle groups were 42.0% and 13.0%, respectively, which is less than those in the anchor localization needle group (55.1% and 23.0%, respectively).
Comparison of pulmonary nodules in different lung bands
Pulmonary nodules in the middle and inner lung bands were grouped together and compared with those in the periphery band, as detailed in Table III. This table highlights the differences in lung nodule size and surgical procedures between the 2 groups. The median diameter of pulmonary nodules in the periphery band group was 0.7 cm, which is smaller than the 1.0 cm median diameter in the middle and inner band group. The primary distinction in surgical procedures is the higher proportion of segmental resections in the middle and inner band group (19.3%) compared to the periphery band group (4.2%).
Table III
Comparison of pulmonary nodules in different lung bands
| Variables | Periphery lung nodule (190 lung nodules) | Middle and inner lung nodule (57 lung nodules) | P-value |
|---|---|---|---|
| Age [years] | 49 (24–80) | 50 (26–75) | 0.529 |
| Tumour size [cm] | 0.7 (0.2–1.8) | 1.0 (0.2–2.0) | < 0.001** |
| Depth to pleura [cm] | 0.6 (0–1.9) | 1.8 (0–4.5) | < 0.001** |
| Puncture location time [min] | 13 (3–30) | 13 (3–26) | 0.564 |
| Alveolar haemorrhage | 104 (54.7) | 39 (68.4) | 0.920 |
| Pneumothorax | 53 (27.9) | 18 (31.6) | 0.618 |
| Imaging morphology, n (%): | 0.212 | ||
| Ground-Glass | 125 (65.8) | 30 (52.6) | |
| Part-Solid | 21 (11.1) | 9 (15.8) | |
| Solid | 44 (23.1) | 18 (31.6) | |
| Surgical procedure, n (%): | 0.002** | ||
| Wedge resection | 175 (92.1) | 44 (77.1) | |
| Segmental resection | 8 (4.2) | 11 (19.3) | |
| Lobectomy | 7 (3.7) | 2 (3.5) |
Localized nodule pathology
According to the WHO 2021 lung tumour histological classification [11], the classification and proportion of surgical pathological results of the 247 pulmonary nodules were as follows: 1) 152 (61.5%) pulmonary nodules were adenocarcinomas, including microinvasive adenocarcinoma and invasive adenocarcinoma; 2) 60 (24.3%) were precursor gland adenoma, including atypical adenomatous hyperplasia and adenocarcinoma in situ; and 3) some pathologies showed only a small number of pulmonary nodules, including 1 case of papilloma, 1 case of bronchial adenoma, 7 cases of pulmonary hamartoma, 3 cases of intrapulmonary lymph nodes, 6 cases of granulomatous lung disease, 2 cases of metastatic tumor, 1 case of minute pulmonary meningothelial-like nodule, and 7 cases of other benign lesions (Table IV).
Table IV
Postoperative pathological results of 247 lung nodules
Discussion
Preoperative localization techniques can be used to quickly find pulmonary nodules that are difficult to locate under VATS. Simultaneously, sublobar resection was performed with accurate localization, and the minimum extent of pulmonary nodules was removed to avoid excessive resection of normal lung tissue and maximize the protection of lung function [12, 13]. As presented in our clinical work, a ground-glass nodule in the upper lobe of the right lung was seen in a middle-aged woman, in whom the VATS was performed directly without preoperative location of the nodule. The nodule was not found in the surgical specimen but was still visible in the postoperative CT scan. Preoperative positioning was performed before the second surgery, and a ground-glass nodule was quickly removed during surgery (Photo 2). This retrospective study demonstrates that the location technique has a high success rate. Indeed, pulmonary nodule localization was stopped in only one of the 216 patients because of a moderate amount of pneumothorax occurring during the localization process, which masked the pulmonary nodule. Additionally, the localization time was short, and a pulmonary nodule can be completed in about 13 min. The complications during location were mild and included needle bleeding and a small amount of pneumothorax, which did not require treatment.
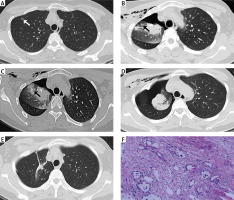
Photo 2
Failed cases of resection of pulmonary nodules under video-assisted thoracoscopic surgery (VATS) without preoperative localization in a middle-aged woman; (A) the CT image showed a ground glass nodule in the right upper lobe; (B) the CT image still showed the presence of pulmonary nodules in the right upper lobe; (C) preoperative localization was performed before VATS again; (D) a second post-VATS CT review showed that the ground glass nodule had been resected on the image; (E) half a year after operation, CT showed that there was no abnormal density shadow in the operation area; (F) the pathological results of the second postoperative surgical specimen showed pulmonary interstitial hyperplasia with focal collagenization
In this retrospective study, 2 pulmonary nodule localization needles were used during the positioning process, the main difference being that the anchor localization needle has 4 hooks. After completion of CT-guidance, the localization needle is pushed out and fixed with the lung tissue using the 4 hooks. However, the breast localization needle has a single hook, which may explain why breast localization needles are prone to detachment. Our statistical results show that the breast localization needle detachment rate was 8.7; however, no detachment occurred in the anchor localization needle, similarly to previous studies [14, 15]. A prior meta-analysis revealed that a high detachment rate is attributed to the structure of the hook-wire [16].
Moreover, in the anchor localization needles, the positioning line is connected to the 4-hook; this positioning line sets different colour segments to more intuitively observe the depth of the positioning nodule. In breast localization needles, the steel wire is connected to a single hook. We evaluated the pain after using the 2 types of pulmonary nodule localization needle according to the VAS score. The pain level of the anchor localization needles was approximately 2, which was significantly lower than that of the breast localization needles, which was approximately 5. Previous studies have reported that the pain level of the anchor localization needles was 2.96 ±1.43 [17].
We also found that the breast localization needle group took longer to locate than the anchor localization needle group. This may be due to issues with proficiency, in that the breast localization needles were used at the beginning of positioning, and the anchor localization needles were used later. The main advantages of the anchor positioning needle are the 4-claw and tri-coloured suture structure, which mean that the anchor positioning needle does not detach and the pain degree of the patient after positioning is reduced. Among the complications, there was no significant difference in the incidence of haemorrhage and pneumothorax between the 2 positioning needles. Although the anchor localization needles have obvious advantages, the price of the anchor localization needles is much higher than that of the breast positioning needle.
In addition to the above-mentioned positioning technology, other commonly used preoperative localization techniques are used in clinical practice, including CT-guided percutaneous coil and liquid material (lipiodol, medical glue, methylene blue)-assisted localization technology, and bronchoscopic-assisted localization technology [10, 18]. CT-guided positioning is easy to operate and takes less time for positioning. Moreover, lipiodol and methylene blue are readily available and inexpensive, and medical glue has good biological safety and firm positioning. However, these techniques also have some limitations, including the following: 1) the coil is the most expensive, approximately 362 USD, while the hookwire and anchor are approximately 55 USD and 250 USD; 2) methylene blue diffuses rapidly and surgery should be performed within 1–2 h of localization; 3) lipiodol positioning in surgery needs to be performed under X-ray fluoroscopy, exposing the surgeon to ionizing radiation; and 4) medical glue has a certain pungent odour, which can cause coughing and allergic reactions in some patients [10]. Studies have shown that the efficacy of bronchoscopic-assisted localization of pulmonary nodules mirrors that of CT-guided localization [19]. However, despite its effectiveness, bronchoscopic-assisted localization technology is rarely applied in clinical practice due to the complexity of its use and high price. In contrast, electromagnetic navigation technology, an innovative technology that may change the field of pulmonary surgery in the future, has been widely used in recent years [18, 20]. Considering the advantages and disadvantages of these various positioning methods, we mainly choose anchor positioning needles in clinical practice.
Interestingly, among the 152 cases of lung adenocarcinoma in our retrospective study, only 8.3% (12/145) had a history of smoking, with female lung cancer accounting for 70.3%, and none of the woman had a history of smoking. We conclude that women who have never smoked account for most cases of lung adenocarcinoma. These results follow previous studies showing that the proportion of non-smoking lung cancer was 74% in women, and 93% of the pathological type was lung adenocarcinoma [21]. Non-smoking lung cancer is generally non-small cell lung cancer, and the most common pathological type is adenocarcinoma [22]. The reason for the high prevalence in current non-smoking female patients is unclear. However, in addition to environmental factors, this may be related to the involvement of oestrogen in the occurrence of lung cancer, or the changes in molecular level and genetic factors [23].
In our retrospective study, pulmonary nodules were predominantly surgically resected using wedge resection. For lung adenocarcinoma nodules with a maximum diameter of ≤ 2.0 cm and a consolidation tumour ratio of < 0.5, the surgical approach can be either segmental or wedge resection [24]. The JCOG0804/WJOG4507L study also concluded that, for these tumours, lung wedge resection can achieve the same prognosis as both segmental resection and lobectomy [25]. The JCOG0802/WJOG4607L study indicated that for stage IA peripheral non-small cell lung cancer with a diameter ≤ 2.0 cm and a consolidation tumour ratio > 0.5, segmental resection and lobectomy offer similar overall survival and postoperative disease-free survival rates. Furthermore, segmental resection was found to be more effective in preserving lung function to the maximum extent [26]. If these tumours are located in the peripheral band, lung wedge resection has a superior outcome compared to segmental resection [27]. Our statistical findings revealed differences in surgical resection methods between the pulmonary nodules situated in the peripheral band group and those in the middle and inner bands. In clinical practice, for pulmonary nodules near the pleura, wedge resection is preferred due to its anatomical advantages. Pulmonary nodules located in the middle and inner bands, which are farther from the pleura, are more amenable to segmental resection because of their anatomical position.
Our results show that only the size of the pulmonary nodules was statistically significant in the total localization pulmonary nodule and lung adenocarcinoma groups. Among pulmonary nodules > 1.0 cm, 88% (37/42) of the localized pulmonary nodules were lung adenocarcinomas. However, only 36% (24/67) of the localized pulmonary nodules ≤ 0.5 cm were lung adenocarcinomas, and most of them were ground-glass nodules. According to the Fleischner Society 2017, the suspected malignant ground glass < 0.6 cm, follow-up was conducted at 2 and 4 years. During the follow-up, surgical resection can be performed if the solid component of the ground glass nodule, the increase in the solid component of the part-solid nodule, or the enlargement of the pulmonary nodule are observed [28]. Therefore, we should be cautious when using resection treatment for nodules < 0.6 cm. Early surgical intervention, in addition to reducing the impact of pulmonary function, also affects the treatment of recurrent nodules. Regular follow-up or CT-guided needle biopsy can be performed to confirm the pathology and decide whether to proceed with surgical resection, to increase the disease-free survival of patients.
Our retrospective study had some limitations. First, all patients examined were treated in a single institution, limiting the generalization of the statistics. Second, there are many methods of positioning, and the study is only a comparison of 2 localization methods, which can be compared with several other location methods.
Conclusions
Unlike breast localization needles, anchor localization needles can reduce the pain and discomfort of patients after positioning, and is not easy to produce decoupling. Both of these localization needles are safe and effective when used under a CT-guided method, which can shorten the time of VATS and accurately remove pulmonary nodules.








