Introduction
Pulmonary nodules (PNs) are non-transparent, ovoid or round lesions with a diameter ≤ 30 mm that are surrounded by the lung parenchyma [1–3]. The establishment of computed tomography (CT)-based screening efforts has led to a marked rise in the rate of PN detection [4]. Differentiating between malignant and benign PNs is generally performed by testing for tumour markers and assessing the morphology, size, and CT density of the target lesions [5–7]. The odds that a given PN is malignant tend to rise with lesion size [8–10], and lesions with a diameter of 5–10 mm exhibit malignancy rates between 47.5% and 61.5% [11–20].
At present, recommendations from the Fleischner Society indicate that solitary solid nodules over 8 mm in diameter or partially solid nodules with solid components greater than 6 mm in diameter should undergo biopsy or surgical resection if persistent [8]. The small size of sub-centimetre PNs (≤ 10 mm), however, makes the CT-guided biopsy of these lesions more difficult. As such, there has been a high degree of variability among published studies employing various needle types, CT methods, and co-axial method use with respect to the diagnostic accuracy (79–98%), pneumothorax (7–62%), and pulmonary haemoptysis (6–22%) rates among patients with sub-centimetre PNs undergoing biopsy procedures [11–20]. There is thus a pressing need for large-scale, systematic analysis of these extant studies in an effort to provide a robust foundation with which clinicians can more effectively evaluate the safety, diagnostic accuracy, and feasibility of CT-guided biopsy approaches such that they can select the most effective biopsy approach.
Aim
This meta-analysis was performed to evaluate the diagnostic performance and safety profile associated with the CT-guided biopsy of sub-centimetre PNs.
Material and methods
Study selection
This study was conducted based on the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) checklist [21] and was registered at inplasy.com (No. INPLASY202350031). Relevant studies published through April 2023 were identified in the PubMed, Web of Science, and Wanfang databases with the search strategy: (((((computed tomography) OR (CT)) AND ((lung) OR (pulmonary))) AND (biopsy)) AND (((1 cm) OR (10 mm)) OR (subcentimeter))) AND ((nodule) OR (lesion)).
Studies eligible for inclusion were as follows: (i) those that were specifically developed to analyse CT-guided biopsy outcomes in patients with sub-centimetre PNs; (ii) studies with a sample size greater than 20; and (iii) studies in which a minimum of one of the technical or clinical endpoints of interest (technical success, diagnostic yield, diagnostic accuracy, pulmonary haemorrhage, and pneumothorax rates) was evaluated. Studies were excluded if they were: (i) reviews, (ii) conference abstracts, or (iii) non-human studies.
Data extraction
Two authors independently extracted relevant data from all studies, and any discrepant results were resolved through discussion with a third investigator. The baseline data for included studies are presented in Table I.
Table I
Characteristics of studies included in the meta-analysis
| Studies | Year | Country/area | Patients number | Mean age | M/F | Mean lesion size | Type of needle | Guidance methods | Co-axial technique | Mean number of samples | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chang [11] | 2018 | Taiwan | 259 | Not given | 111/148 | Not given | Core | CT | Yes | Not given | 8 |
| Choi [12] | 2013 | Korea | 268 | Not given | Not given | 9.3 mm | Core and Fine | CT | No | 2.0 | 7 |
| Choo [13] | 2013 | Korea | 105 | 62 y | 55/50 | 8.5 mm | Core | CBCT | Yes | Not given | 8 |
| Dominguez-Konicki [14] | 2020 | USA | 193 | 65.5 y | 114/79 | Not given | Core and Fine | CTF | Yes | Not given | 8 |
| Hui [15] | 2022 | China | 105 | 58.9 y | 57/48 | 9.1 mm | Core | CT | No | 1.7 | 8 |
| Hwang [16] | 2018 | Korea | 213 | 62.1 y | 99/114 | 9 mm | Core and Fine | CBCT | Yes | Not given | 8 |
| Li [17] | 2020 | China | 101 | 57.8 y | 55/46 | 9 mm | Core | CT | No | 1.7 | 8 |
| Ng [18] | 2008 | Canada | 54 | 63.3 y | 28/26 | 9 mm | Fine | CT | Yes | Not given | 8 |
| Portela de Oliveira [19] | 2020 | Canada | 127 | 65.4 y | 65/62 | 9.2 mm | Core and Fine | CT | No | Not given | 8 |
| Wallace [20] | 2002 | USA | 57 | 61.3 y | 30/27 | Not given | Fine | CT | No | Not given | 7 |
Technical success for CT-guided biopsy procedures was defined by the successful collection of a sample of quality that was adequate to permit visual inspection [15]. Diagnostic yield was assessed by dividing the diagnostic results based upon CT-guided biopsy procedures by the total results [15]. Diagnostic accuracy was calculated based on the sum of the numbers of true positive and true negative results [15].
Quality assessment
The quality of non-randomized controlled trials was assessed with the Newcastle-Ottawa scale (NOS) [10]. These studies were scored based on selection, comparability, and outcome criteria, which were awarded up to 4, 2, and 3 points each, respectively. Studies with a total NOS score of 7 or higher were considered to be of high quality.
Statistical analysis
Random-effects models were used to evaluate pooled data for all study endpoints. Pooled result heterogeneity was assessed using the Q test and I2 values, with significant heterogeneity being defined by an I2 value > 50%. Sensitivity analyses were performed with a leave-one-out approach, and subgroup analyses were performed based on guidance methods, needle types, and whether a co-axial method was employed. Egger’s test was used to assess the potential for publication bias, and p < 0.05 was the cut-off for statistical significance. Stata 12.0 was used to conduct all pooled analyses.
Results
Study selection
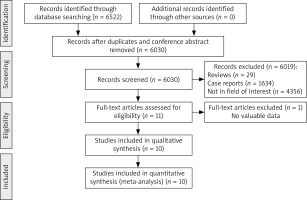
Of 6522 initially identified studies, 10 were ultimately incorporated into this meta-analysis, as detailed in Figure 1. These studies included a total of 1482 patients undergoing CT-guided biopsy procedures for the evaluation of sub-centimetre PNs (Table I). Biopsies in 4, 2, and 4 of these studies were performed using core needles, fine needles, or both needle types, respectively. In addition, 7, 2, and 1 of these studies employed conventional CT, cone-beam CT (CBCT), and CT fluoroscopy (CTF) guidance, respectively, while the co-axial technique was employed for the biopsy procedures in 4 of these studies. All these studies were of high quality, with NOS scores ranging from 7 to 8.
Technical success
Technical success rates were reported in 3 studies [12, 17, 20], with a pooled technical success rate of 90% (95% CI: 0.81–0.99, Figure 2 A). Significant heterogeneity was detected (I2 = 93.6%), but there was no evidence of publication bias (p = 0.247). Sensitivity analyses failed to establish the sources of heterogeneity for this endpoint.
Diagnostic yield
Diagnostic yield was reported in 4 studies [14, 15, 17, 19], with an overall pooled yield rate of 60% (95% CI: 0.41–0.79, Figure 2 B). Both significant heterogeneity (I2 = 96%) and publication bias (p = 0.008) were detected, but sensitivity analyses failed to establish the sources of heterogeneity for this endpoint.
Diagnostic accuracy
Diagnostic accuracy rates were reported in 8 studies [11–13, 15–18, 20], with an overall pooled rate of 91% (95% CI: 0.88–0.95, Figure 2 C). Both significant heterogeneity (I2 = 76.9%) and publication bias (p = 0.001) were detected, but sensitivity analyses failed to establish the sources of heterogeneity for this endpoint.
Pneumothorax
Pneumothorax rates were reported by 8 studies [11–13, 15–17, 19, 20], with an overall pooled incidence rate of 24% (95% CI: 0.16–0.33, Figure 2 D). Significant heterogeneity was detected (I2 = 93.6%), but there was no evidence of publication bias (p = 0.656). Sensitivity analyses failed to establish the sources of heterogeneity for this endpoint.
Pulmonary haemorrhage
Pulmonary haemorrhage rates were reported by 6 studies [11, 13, 15–17, 19], and the pooled rate was 11% (95% CI: 0.06–0.16, Figure 2 E). Significant heterogeneity was detected (I2 = 80.8%), but there was no evidence of publication bias (p = 0.942). Sensitivity analyses identified the study conducted by Chang et al. [11] as the source of detected heterogeneity.
Subgroup analyses
The results of subgroup analyses conducted for diagnostic accuracy rates are compiled in Table II. The respective pooled rates for patients who underwent fine-needle and core-needle procedures were 85% and 95% respectively, while the respective rates for patients who underwent biopsy procedures performed with conventional CT and CBCT guidance were 92% and 95%, and the pooled rates for patients that underwent biopsy procedures that did and did not utilize the co-axial method were 94% and 93%, respectively.
Table II
Subgroup analyses of diagnostic accuracy
The results of subgroup analyses conducted for pneumothorax rates are compiled in Table III. The respective pooled rates for patients who underwent fine-needle and core-needle procedures were 62% and 15%, respectively, while the respective rates for patients who underwent biopsy procedures performed with conventional CT and CBCT guidance were 22% and 15%, and the pooled rates for patients that underwent biopsy procedures that did and did not utilize the co-axial method were both 20%.
Table III
Subgroup analyses of pneumothorax
The results of subgroup analyses conducted for pulmonary haemorrhage rates are compiled in Table IV. Respective pooled pulmonary haemorrhage rates for patients who underwent biopsy procedures performed with conventional CT and CBCT guidance were 12% and 9%. Respective pooled rates for patients who underwent biopsy procedures that did and did not utilize the co-axial method were 12% and 9%.
Discussion
The present meta-analysis examined the diagnostic accuracy, feasibility, and safety of using CT-guided biopsy as an approach to the diagnosis of sub-centimetre PNs. The 90% pooled rate of technical success computed for the included studies suggests that CT-guided biopsy is a reliable means of collecting samples from sub-centimetre PNs that are large enough to permit pathological examination. This pooled rate, however, was below the 99–100% rates that have been reported in other analyses focused on the CT-guided biopsy of PNs [6, 22, 23]. Of the studies incorporated into this meta-analysis, the article published by Wallace et al. [20] reported a low 77% technical success rate, whereas the other studies reporting this endpoint exhibited rates greater than 90% [12, 17]. This may be attributable to the fact that the CT-guided biopsy procedures included in the Wallace et al. study were conducted from 1999 to 2001, and technical success rates may have improved over time with advances including the development of CT multiplanar reformation, CTF, and CBCT approaches.
In this meta-analysis, the pooled diagnostic yield rate indicated that CT-guided biopsy procedures alone can yield a definitive diagnosis in roughly 60% of patients being evaluated for sub-centimetre PNs, in line with rates of 65–71% that have previously been reported for the CT-guided biopsy of PNs [22, 24].
In this meta-analysis, the pooled diagnostic accuracy rate for CT-guided biopsy procedures was 91%, demonstrating the value of this approach as an accurate means of diagnosing sub-centimetre PN diagnosis. In subgroup analyses, the use of guidance methods (conventional CT vs. CBCT) or the co-axial method had no impact on this diagnostic accuracy rate. However, a notable difference in this rate was evident when comparing fine- and core-needle biopsy procedures (85% vs. 95%). Supporting this finding, core-needle biopsy procedures have been demonstrated to achieve sample adequacy superior to that associated with fine-needle biopsy procedures [25]. Core-needle biopsy procedures are also better suited to the evaluation of specific molecular markers of interest, providing supplemental information that can support traditional pathological diagnostic findings [26].
Rates of pulmonary haemorrhage and pneumothorax were analysed as a metric to gauge the safety of CT-guided biopsy procedures in patients with sub-centimetre PNs. The respective pooled incidence rates of these 2 complications were 11% and 24% among the included studies, in line with similar rates reported in a prior meta-analysis focused on the CT-guided biopsy of small PNs (≤ 20 mm) [27]. Some prior meta-analyses have reported no differences in rates of pulmonary haemorrhage or pneumothorax as a function of needle type (core vs. fine needle) or guidance methods used (conventional CT vs. CTF) [25, 28]. While the subgroup analyses conducted herein indicated that the pneumothorax rate associated with fine-needle biopsy procedures was 62%, this result was only derived from a single report [20], highlighting the need for further research to better gauge this procedural risk.
There are certain limitations to this meta-analysis. Firstly, all included studies were retrospective in design and may thus be associated with a higher risk of bias, emphasizing a need for prospective validation. Secondly, only single-arm results were taken into consideration in this study, precluding any potential comparisons between 2 different methods. And thirdly, some of the included studies utilized 2 different needle types and did not report which needles were used for which patients, introducing potential bias into the subgroup analyses conducted based on needle type.