Introduction
The pharmacological control of the secretion of gastric acid is one of the main strategies employed in managing various gastric complications such as various types of ulcers, eradication of Helicobacter pylori, Zollinger-Ellison syndrome, erosive oesophagitis, non-erosive reflux disease, and functional dyspepsia. This inhibition is accomplished most viably with proton pump inhibitors (PPIs). Over the past decades, the use of proton pump inhibitors has increased gradually. This increase in the consumption of PPIs is also enhanced by gastroduodenal side effects associated with polypharmacy along with other standard indications [1]. A study demonstrated that PPIs are overprescribed medications, both in primary and secondary care settings in Karachi, Pakistan. An estimated 47.2% of the discharged patients were prescribed PPIs during the study duration [2]. In another study, conducted on 1800 patients in 24 weeks, who were admitted to medical wards and emergency departments, showed that 72.6% of the patients had been prescribed PPIs in Lahore [3]. Similarly, other studies have demonstrated the overutilisation of PPIs in different parts of the globe [4–6].
Even though PPIs have an admirable safety profile in short-term therapy, with time, the increasing use of PPIs in terms of treatment duration has raised serious concerns about the risks to patients. Patients take unnecessary PPIs for extended periods, whereas they should utilise PPIs in a minimal dose for a short duration. Regular use of PPIs may be useful for some patients, but that is also time-bound. The inappropriate use of PPIs has also been the subject of studies. In various studies, the use of PPIs has been shown against well-defined indications mentioned in the Food and Drug Administration (FDA) and the National Institute for Health and Care Excellence (NICE) guidelines [7, 8]. Treatment protocols in terms of the duration of PPI therapy for some of the indications are outlined here:
for active duodenal ulcers, PPIs can be prescribed initially for 4 weeks. Some patients may require additional 4-week therapy.
for benign gastric ulcers PPIs therapy can be given for 4–8 weeks.
for treating symptomatic gastroesophageal reflux disorders (GERD), 4 weeks of PPIs therapy is required.
for erosive oesophagitis, a 4–8-week therapy is beneficial.
The updated NICE guidelines for PPI therapy recommend a maximum of 8 weeks of treatment, while in FDA guidelines for some clinical scenarios like gastric ulcer and risk reduction associated with non-steroid anti-inflammatory drugs (NSAID), PPIs like lansoprazole can be given up to 12 weeks [9, 10]. The FDA guidelines do not recommend a 14-day PPI therapy more than 3 times in a single year [11]. Apart from the above, the maximum treatment duration for PPIs beyond 12 weeks in a single continuous therapy has not been documented in any standard guidelines. There is some evidence that PPIs are being used irrationally, and some patients continue PPI treatment for several years without justification for continuous therapy [7, 12].
Aim
The aim was to evaluate the frequency of continuous long-term PPI users in selected outpatient clinics in Khyber Pakhtunkhwa, Pakistan.
Material and methods
A cross-sectional study was conducted in 2 gastroenterology outpatient clinics in Khyber Pakhtunkhwa (K.P.) from January 2018 to November 2019. Ethical approval (certificate No. PHM.Eth/CF- M10/17-0042) for the study was taken from the Ethical Committee of the Department of Pharmacy, COMSATS University Islamabad, Abbottabad Campus, and the Medical Ethics Committee of the Medical Teaching Institute, Abbottabad. Written consent was taken from all participating patients visiting the gastroenterology outpatient clinics for different gastrointestinal tract (GIT) complications. Subject participants were enrolled in the study by nonprobability consecutive sampling. The sample size was calculated using the Cochrane formula: n = Z2 p(1 – p)/d2, where n is the desired sample size, Z is the statistics corresponding to the confidence level (95%), p is the expected prevalence (50%), and d is the relative precision/margin of error (7.5%). The estimated sample size calculated for our study was n = 171.
Patients were interviewed based on the duration of treatment with PPIs to extract the history of PPI use. A cut-off period of 3 months (more than 12 weeks) of continuous PPI administration was chosen for inappropriate PPIs use. Demographic data including age, sex, family income, marital status, and family members using PPIs were collected. The data relating to PPI use included the type of PPI, dose, frequency, duration of PPI use, and other concomitant drugs. In patients using PPI for many years, the criteria for the selection of continuous PPI users were considered as the usage of PPI at least 3 times a week once daily (OD) or using it every alternate day.
Results and Discussion
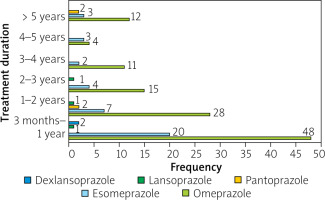
The demographic data showed that most of the patients were female (60.8%, Table I). The mean age of the patients was 42.46 ±14.21 years with a range from 16 to 80 years. The duration of PPI treatment within each age group was also evaluated (Figure 1). A high proportion of PPI users were in the 35-44 years age group followed by 25–34 years and 45–54 years, respectively.
Table I
Demographics and summary of results
In all patients, PPI was initially prescribed by a physician. An estimated 110 (66.27%) patients had a physician’s advise for long-term continuous PPI use, and the remaining one-third 56 (33.73%) patients were using PPI beyond the main treatment course on their own without proper prescription for continuous long-term use. The duration of PPI use was in the range of 3 months to 15 years. Around 95 (57.2%) out of 166 patients were using PPI for more than 1 year. 42.77% of patients were using continuous PPI with a time duration of over 3 months to 1 year followed by 38 (22.89%) patients with PPI use within a range 1–2 years. A considerable proportion of 17 (10.24%) patients were observed who were using PPI for more than 5 years (Table II). In addition, around 35% of the study population indicated that at least one of their family members used PPIs. Seventeen (10.24%) patients were using PPI in combination with prokinetics and H2 receptor blockers, among which 8.43% of patients were using prokinetics and 1.81% of patients were using H2 receptor blockers along with PPI. Among various PPIs, omeprazole appeared to be the drug of choice with the highest usage by 71% of patients.
Table II
Duration and types of PPIs use
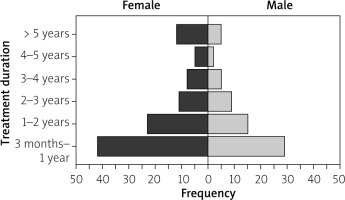
The frequency of long-term inexpedient use in male and female patients is shown in Figure 2. The frequency of inappropriate PPI use was higher in female patients than in male patients.
The frequency of different PPI use over time was evaluated and is shown in Figure 1. In total, 42.77% of patients were using continuous PPI with a time duration of over 3 months to 1 year followed by 22.89% of patients with PPI use within a range from 1 to 2 years. A significant proportion of 10.24% of patients were observed who were using PPIs for more than 5 years.
Omeprazole and esomeprazole are the leading PPIs used continuously for a longer duration. An estimated 10.16% of the patients were receiving PPI in BID frequency (Table III). Similarly, 27.96% of the omeprazole users were taking a 40 mg dose, 33.33% of the esomeprazole users were receiving a 40 mg dose, while all pantoprazole and dexlansoprazole users were taking 30 mg and 60 mg daily doses, respectively.
Table III
Dose and frequency observed in PPI user of ≥ 3 months
| PPI type | Dose frequency | Dose strength | ||
|---|---|---|---|---|
| BID | OD | 20 mg | 40 mg | |
| Omeprazole | 12 | 106 | 85 | 33 |
| Esomeprazole | 4 | 35 | 26 | 13 |
| Pantoprazole | 0 | 4 | 0 | 4 |
| Lansoprazole* | 0 | 3 | 3 | 0 |
| Dexlansoprazole** | 0 | 2 | 0 | 2 |
| Total | 16 | 150 | 114 | 52 |
A significant proportion of PPI users were using a maximum daily dose in all age groups (Table IV). A considerable proportion of BID users of PPI were also documented in all age groups.
Table IV
Use of PPIs in various age groups
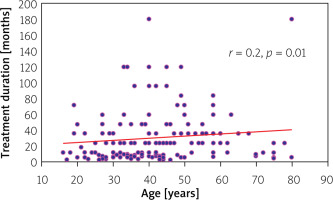
Correlation analysis was used to calculate the association between treatment duration and the age of patients (Figure 3). The analysis indicated a weak positive correlation (r = 0.2), which was statistically significant (p = 0.01).
Kruskal-Wallis and Mann-Whitney U tests were carried out, and significant differences were observed between the age groups of participants and the duration of PPI use (p = 0.01). However, no significant association was found between gender and the duration of PPI use (p = 0.516) (Table V).
Table V
Association of the duration of PPI use with respondents’ demographics
Proton pump inhibitors (PPIs) are commonly used drugs for multiple gastrointestinal complications. It is commonly used in both hospitalised and outpatients. However, little is known about its usage in ambulatory patients in the K.P.K region. Considering this, the present study was designed to evaluate the utilisation pattern of PPIs.
Our demographic data showed that PPI consumption was high in female compared to male patients. It appears that either this gender is inclined toward gastric complications or polypharmacy, an area worthy of further investigation. Furthermore, various guidelines suggest the use of PPIs for 4–8 weeks. However, our data indicate that consumption of PPIs for more than 3 months is highly prevalent. The findings of this study are consistent with the others cited in the literature [7, 12]. Importantly, regular usage of PPIs for up to 15 years was also noted in the present study. Like other drugs, the use of PPIs is not without its side effects, and such a pattern of use will put the patient’s overall health at greater risk. Numerous studies have shown that long-term treatment with PPIs may impose many complications such as clostridium difficile-associated disease, community-acquired pneumonia, dementia, kidney diseases, anaemia, and nutrient deficiency such as vitamin B12, magnesium, calcium, and iron [13–15]. Furthermore, a study has shown an association between chronic PPI use and the risk of developing gastric cancer [16]. In most cases, patients are completely unaware of these harmful effects, and many patients continue to use regular PPIs after their first prescription. This unawareness leads to the increased and inexpedient use of PPIs. The poor implementation of pharmacy laws and the ill-managed regulatory system in Pakistan provides easy access to prescription drugs. Unauthorised medical practitioners and quacks also contribute to this inexpedient use of PPI [17]. The availability of some of the cheap brands further adds to the inexpedient use of PPI as patients often take such medications for very minor symptoms that can be simply resolved through patient lifestyle changes. Dose tampering and other alternative medications such as antacids and H2 receptor blockers are useful in managing rebound symptoms [18–20].
The results of this study indicate that omeprazole and esomeprazole are the leading PPIs used by patients. Both account for 94.6% of the PPIs used by our study population, in which omeprazole accounted for 71.1% and esomeprazole 23.5%. In the remaining 5.6% of the study population, other PPIs were used. These results are synonymous with a study conducted in other areas of Pakistan [2]. Omeprazole has been reported as the second-largest molecule by sale value in the pharmaceutical sector in Pakistan, indicating its widespread use [21]. Although there is extensive variation in the per-dose cost of different brands of PPIs available in Pakistan, the average cost of omeprazole and esomeprazole are lower than for other PPIs, which further adds to their selection in the self-prescribed scenario.
Analysis of inappropriate use of PPIs in terms of their dose and dosing frequency showed that 68.7% of patients were using PPIs in 20/30 mg at a single time and the remaining 31.32% were using PPIs with 40/60 mg dose regularly for a longer duration. Similarly, 9.6% of the patients were using PPIs in BID frequency. Within age groups, the 45–54 age group received the maximum daily dose, while higher BID frequency was observed in younger patients. All PPIs differ in their relative potencies, and there may be better therapeutic chances for higher doses or twice-daily regimens compared to normal routine doses. One should consider the choice of PPI regimen and dosing based on the indication for use, and the lowest effective dose should be used to restrict the adverse effects related to PPI use [22].
Our findings suggest that with an increase in age, the chance of long-term use increases. These findings agree with that of the study conducted by Vilcu et al. [23] in which they identified a significant association between PPI use and older age groups (45–64 years), who were also at highest risk, while younger age groups (0–14 years and 15–44 years) were at no significant increased risk. In addition, our findings also indicate that the prevalence of long-term regular use in the younger population is also considered, which makes them at higher risk for developing adverse effects. We also identified a major concern within our study population related to PPI use in geriatric patients. In our study, 7.2% of the study population were of age 65 years and above and were regularly using PPIs for a longer duration ranging from 5 months to more than 5 years. According to Beer’s criteria 2019, all PPIs are listed as potentially inappropriate medications for older adults due to their risk for clostridium difficile infection and bone loss and fracture in the older population. Some other common adverse effects that are associated with the long-term use of PPIs have been discussed above. According to Beer’s criteria recommendations, the scheduled use of PPI for more than 8 weeks should be avoided unless for high-risk patients, i.e. chronic NSAID and oral corticosteroids users, Barrett oesophagitis, erosive esophagitis, pathological hypersecretory conditions, or a demonstrated need for maintenance treatment (because of failure of drug discontinuation trial or H2 receptor antagonists) [24]. Concerns about the unauthorised use of PPIs have been growing, and in many developed countries, efforts are being taken to stop or slow down the inexpedient use of PPIs, and they have initiated deprescribing plans. In some instances, various guidelines have also been developed and are being practiced [25, 26]. In a developing country like Pakistan, the consequences of such overuse of PPIs may be more dangerous in the future; therefore, unified efforts are needed from every stakeholder in overcoming the overuse of PPIs.
Conclusions
The present study demonstrated that PPIs are being used inexpediently by both young and elderly patients for a longer duration beyond the regular treatment course in outpatient clinics. Based on these findings, it is suggested that a proper evaluation regarding PPI use is essential for the clinical management of such patients in outpatient clinics. The patient must be counselled properly regarding PPI therapy, dose, frequency, duration of treatment, stepping down PPI, and the management of rebound symptoms.