Introduction
Hepatocellular adenoma (HCA) is one of the benign liver tumors. It requires differentiation from focal nodular hyperplasia (FNH), hemangioma, and hepatocellular carcinoma (HCC), to which it can progress. In most cases it requires long-term observation due to its metaplastic potential. HCA can succumb to rupture and should be monitored especially in the case of young women, because of the correlation of occurrence with use of oral contraceptives [1-3]. FNH should also be observed due to the possibility of growth and potential compression of surrounding tissues and organs. Diagnosis of the tumors mentioned above should be made using magnetic resonance imaging (MRI)/computed tomography (CT) [4]. The current European Association for the Study of the Liver (EASL) recommendations suggest that biopsy is indicated only in the case of suspicion of malignant transformation [5]. In the aspect of initial diagnosis, contrast-enhanced ultrasound (CEUS) is not included in the recommendations, and at this moment it can be inefficient due to the possibility of overlooking some pathological changes apart from the main lesion. However, it can be used as a great way of monitoring potential evolution of the tumors and providing appropriate biopsy guidance. Our study concerns MRI and CEUS. We wanted to show that CEUS can be useful in monitoring focal liver lesions as well as MRI/CT due to the similar image of lesions as in the above-mentioned methods simultaneously with lower cost of examination and better availability and safety [3]. The aim of the study was to compare the size of tumors, their perfusion pattern, time of enhancement and enhancement homogeneity in MRI with CEUS.
Material and methods
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Bioethics Committee at the Medical University of Łódź (RNN/266/22/KE). All the patients gave informed consent to participate in the project. The inclusion criterion was the confirmation of focal liver lesions using MRI. The CEUS was performed up to 48 h after MRI. The exclusion criteria were in line with the contraindications to use the contrast in ultrasonography according to the recommendations of the SonoVue producer: respiratory insufficiency, acute coronary syndrome, adverse post-contrast reactions, or declaration of pregnancy.
Patients
A retrospective study was performed in the Department of Radiology of the Medical University of Łódź. The study included cases of 47 patients (40 women and 7 men aged 19 to 62) with clinical diagnosis of HCA (32 tumors) and FNH (27 tumors). Three HCA were observed in five patients and two FNH were observed in two patients (in this case, each lesion was considered as a separate observation). The study included the characteristics of the lesion in MRI, which were compared with CEUS. Both procedures were performed with a time interval of not more than 48 hours. 1.5 and 3 T scanners (Siemens Magnetom Avanto, Siemens Magnetom Vida) were used. The MRI examinations were performed according to the LI-RADS Version 2018 [6], taking into account post-contrast sequences with the assessment of late enhancement (0.1 ml of Gadovist 1.0 per kg body weight). Every lesion diagnosis was confirmed by MRI. The size of the lesion was assessed in the early post-contrast phase. The differentiation between HCA and FNH was based on the presence of a central scar (Fig. 1A-D, Fig. 2A-D).
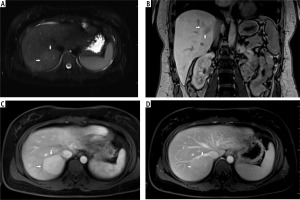
Fig. 1
A) T2-weighted image in transverse plane, focal lesion in right lobe of liver (full white arrows). Signal of lesion slightly higher in relation to the liver parenchyma. In the central part, a small area with lower signal (empty white arrow). B) T1-weighted image in coronal plane, focal lesion in right lobe of the liver (full white arrows). The lesion signal is lower in relation to the liver parenchyma. In this sequence, a well-visible hyposignal central fibrous scar and spoke spaced connective tissue septa are clearly visible. C) T1-weighted image at an early stage after administration of contrast agent. Acquisition in the transverse plane, focal lesion in the right lobe of the liver (full white arrows). The lesion is enhanced intensively in relation to the liver parenchyma. Hyposignal scar remains in the center of the lesion. D) T1-weighted image in the late phase after administration of contrast agent. Acquisition in transverse plane. Focal lesion in the right lobe of the liver (full white arrows). Enhancement of the lesion and the liver parenchyma in this phase is similar. Lesion was qualified as focal nodular hyperplasia (FNH)
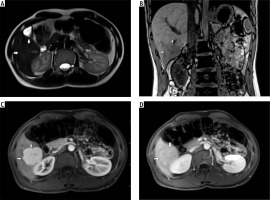
Fig. 2
A) T2-weighted image in transverse plane, focal lesion in the right lobe of the liver (full white arrows). Signal of the lesion slightly higher in relation to the liver parenchyma. B) T1-weighted image in coronal plane. Focal lesion in right lobe of the liver (full white arrows). The signal of the lesion is lower in relation to the liver parenchyma. In this sequence, the heterogeneity of the signal within the lesion is clearly visible. C) T1-weighted image in the early phase after administration of a contrast agent. Acquisition in the transverse plane. Focal lesion in the right lobe of the liver (full white arrows). The lesion enhances intensively in relation to the liver parenchyma. D) T1-weighted image in the late phase after administration of a contrast agent. Acquisition in transverse plane. Focal lesion in the right lobe of the liver (full white arrows). Enhancement of the lesion and the parenchyma of the liver in this phase is similar. The lesion was classified as hepatocellular adenoma (HCA)
Imaging technique
Before CEUS MRI examination in the standard abdominal protocol with contrast was performed for confirmation of focal liver lesion diagnosis. The CEUS was performed according to the guidelines for CEUS in liver – 2020 update [7]. The GE logiq 7 system with convex probe 4C was used. Examination included standard grey scale (B-mode) ultrasound examination of the liver. This part of the examination was performed to evaluate the presence of potential lesions which do not exhibit contrast enhancement and could be misdiagnosed as malignant infiltration. The size, localization, and quantity of lesions were recorded. Next, color Doppler imaging was performed. In the last step CEUS was performed starting with an injection of 2.4 ml of contrast agent (SonoVue) in the medial cubital vein. This dosage was usually sufficient to state a correct diagnosis. An additional dose of contrast agent was administered when needed. The CEUS was conducted using a low mechanical index (< 0.1) to avoid destruction of the contrast agent bubbles [8, 9]. Three main phases of acquisition were performed. The acquisition of each of the phases was appropriate in the following intervals: the arterial phase (10-45 s), the portal venous phase (45-120 s), the late venous phase (120-180 s) [7]. The dynamic enhancement profile change was not noticeable after 120 s of examination. Moreover, the examination of lesions located deep under the diaphragm required deep inhalation and breath-holding, posing challenges for some patients and making the extended acquisition difficult. Extended acquisition was only recorded in cases that were doubtful. Therefore, we decided to present our analysis in the 0-180 s range for clarity in interpreting the study results. During the examination, the enhancement of the tumor was compared to liver parenchyma (Fig. 3, 4).
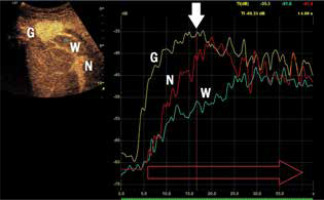
Fig. 3
Assessment of the degrees of enhancement in the post-contrast examination (CEUS). Areas of interest were placed within the lesion (depending on the size of the lesion and the uniformity of the enhancement – one or two areas – G). Another area was placed in the parenchyma of the liver – W, and in the lumen of a large vessel (most often vessels of the portal circulation – N). Enhancement curves were recorded in about 2-3 min in sequences of about 20-30 s. Values burdened with motor artifacts causing the dislocation of areas of interest were eliminated
Statistical analysis
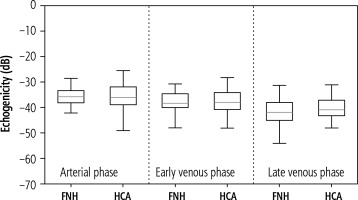
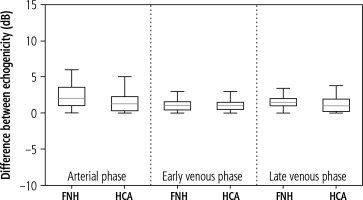
A statistical analysis of the demographic data and size of the lesions was performed. The Shapiro-Wilk normality test was used to assess the distribution of the recruited population. Due to non-normal distribution of continuous variables, differences between the patients’ characteristics were assessed using the Mann-Whitney U test. Next, the case-by-case HCA and FNH enhancements in the CEUS were analyzed in 10 s intervals for a total observation time of 180 s. If a case had a single missing observation, a linear interpolation was used to fill in the gaps. Missing observations were caused by respiratory movement during the acquisition. The mean enhancement values for each tumor over time were calculated. A single-case HCA and FNH enhancement was also presented. The unchanged liver parenchyma enhancement was assessed and compared with the tumor-changed organ. The acquisition of the echogenicity of the marked regions was measured in every phase of the examination – arterial phase, portal venous phase and late venous phase. The distributional characteristics of the difference between the best and the worst part of the tumor are presented in a box plot. The median is marked by the orange line in the center of the box. No outliers were present in the collected data. All the graphs were created using Python matplotlib v3.6. A p-value less than 0.05 was considered statistically significant.
Results
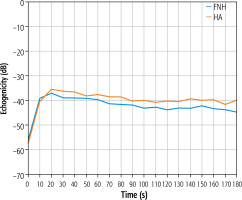
The estimated mean size of the lesions based on post-contrast sequences in MR examinations ranged from 3.29 × 2.75 cm in the adenomas group and from 4.25 × 3.20 cm in the FNH group. The central scar in this group was located eccentrically in only one case, and in the remaining cases it was found in the central part of the tumor. The estimated mean size of the lesions based on post-contrast sequences in the CEUS studies ranged from 3.33 × 2.72 cm in the adenomas group and 4.17 × 3.12 cm in the FNH group (Table 1). The performed statistical testing of the demographic data and size of the lesions demonstrated that the data within the benign lesion groups was similar. The central scar was located in the same way. In both groups, a similar tumor enhancement profile was observed compared to unchanged liver parenchyma (Fig. 5A-C, Fig. 6A-C). In the HCA group, the difference in the arterial phase reached 22 dB (values ranged from 7 to 32 dB). After 20-30 s, the enhancement of the focal lesion was similar to that of the liver parenchyma. In the FNH group, the difference in the arterial phase was also significant, amounting to 20 dB (values ranged from 7 to 28 dB). Already after 20-30 s, the enhancement of the focal lesion was similar to the enhancement of the liver parenchyma. The mean values of enhancement for both groups are presented in Figure 7. In both groups after 20-30 s enhancement of the focal lesion was similar to the liver parenchyma. The values remained comparable until the end of observation (tumor-background gain difference was ±7 dB) (Fig. 8). The difference between the best and the worst enhanced part of tumors did not exceed 3 dB in the FNH group and 2 dB in the HCA group (Fig. 9).
Table 1
Patient demographics and lesion size analysis
Fig. 5
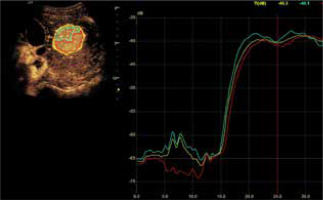
A) Image of ultrasound and color Doppler in the transverse plane, focal lesion in the right lobe of the liver identified in MRI as HCA (full white arrows). Echogenicity of the lesion is slightly higher in relation to the liver parenchyma. B) Image of CEUS in the transverse plane of the lesion identified in MRI as HCA (full white arrows). Enhancement in the early phase is higher in relation to the liver parenchyma. C) Image of CEUS. Curves of enhancement in early phase of examination. The tumor area – yellow and blue lines, enhancement appears in the hepatic veins with slight delay – the green line. Weaker and delayed enhancements in the liver parenchyma – the red line. Disturbance of the curves in the early phase of the study (0-5 s) is caused by displacement of the liver due to breathing. D) Image of CEUS. Curves of enhancement in the areas of interest in the late phase of the study are aligned. The tumor area – blue and yellow line, the hepatic vein is slightly more intensively enhanced – blue line. Slightly weaker enhancement of the liver parenchyma – the red line
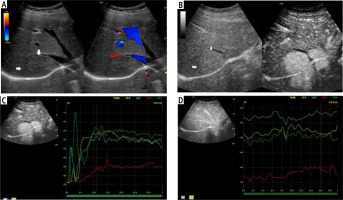
Fig. 6
A) Image of ultrasound and color Doppler in the transverse plane, focal lesion in the right lobe of the liver identified in MRI as FNH (full white arrows). Echogenicity of the lesion is slightly higher in relation to the liver parenchyma. In the central part, a small scar (an empty white arrow) is more clearly visible in the color Doppler option. B) Image of CEUS in the transverse plane of the lesion identified in MR as FNH (full white arrows). Enhancement in the early phase is higher in relation to the liver parenchyma. In the central part, the area containing the vessels, corresponding to the central fibrous scar. C) Image of CEUS. Enhancement curves in the early phase of examination. The tumor area – yellow and blue lines, with slightly delayed enhancement appears in the portal system – red line. Weaker and delayed enhancement of the liver parenchyma – the green line. D) Image of CEUS. Curves of enhancement in late phase of examination align. Area of tumor – blue and red lines, slightly stronger enhancement portal vein – yellow line. Slightly weaker enhancement of the liver parenchyma – the green line
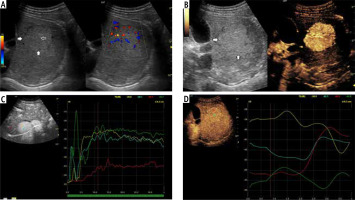
Fig. 7
Mean values of the difference in tumor enhancement and unchanged liver parenchyma over time recorded in the HCA group (blue line) and in the FNH group (red line)
Discussion
Focal nodular hyperplasia is the second most common liver tumor, usually discovered incidentally by ultrasonographic examination of the abdomen [8]. Clinically, the majority of the cases are asymptomatic except when the size of the lesion causes compression of the surrounding tissues. FNH is a limited, usually single, sometimes multiple neoplasm build of normal hepatocytes separated by a fibrous septum. There is a tendency to observe a fibrous scar in the center of the lesion [4, 10]. Some authors correlate the presence of HCA and FNH with oral contraceptives (OC) [1, 2, 10-12]. Our results from MRI and CEUS examination suggest that both FNH and HCA have a similar perfusion pattern and size when measured in both methods. The main feature which allows them to be distinguished is a central fibrous scar, but it tends to be located outside of the center of the lesion. Additionally, in up to 37.5% of FNH cases a central scar is found [13, 14]. Such a lesion, called atypical FNH, makes the diagnosis more cumbersome, especially in smaller tumors. Benign liver tumors are characterized by homogeneous enhancement of the whole tumor during contrast examination. The proposed methodology of analysis in our study shows that enhancement of both lesions during CEUS examination is similar and does not exceed 5 dB. Inhomogeneous structures of the tumor can suggest the potentially malignant character of the lesion. Furthermore, another important feature in diagnostics of focal liver lesions is the presence of the wash-in and wash-out effect. In the case of HCA potential changes of perfusion pattern should be monitored. When the wash-out effect appears, progression to HCC can be suspected. Both diagnostic tools used can show the presence of a central fibrous scar, and wash-in and wash-out effects and homogeneity can be assessed. The obtained enhancement pattern was similar in both types of tumors. According to the EASL guidelines, the first diagnosis should be based on MRI or CT to exclude the presence of other pathological lesions in the liver. Nowadays, MRI is considered superior to other techniques and the patients diagnosed with HCA should be followed up with MRI 6 months after the diagnosis. Then, the modality should include the gender, size and pattern of progression. We propose supervision using CEUS only, especially when the monitored lesion starts to present inhomogeneous enhancement, because this method allows direct biopsy of the suspected region to be performed. Our study shows that every feature suggesting potential malignant transformation of a benign lesion can be observed as loss of enhancement homogeneity. Our study is the first one to address the homogeneity of enhancement of hepatic tumors assessed. Features which should be especially observed include size, homogeneity, wash-in and potential occurrence of a wash-out effect. Furthermore, curves of enhancement marked out using CEUS show alignment of enhancement of the tumor in 20-30 s of examination. If deviations of curves of enhancement are observed in examination, they should also be checked. When some of the features mentioned above seem to be changing, MRI examination is desirable. We found that CEUS outweighs MR due to its easy access and lower costs. Furthermore, it provides continuity of monitoring of contrast distribution during acquisition. In addition, a much lower dose of contrast agent allows CEUS to be used in patients with renal failure. Furthermore, metal implants or claustrophobia does not disqualify the patient from examination. Other ways to discriminate focal liver lesions are biopsy and tumor markers. In the case of biopsy, localization of the tumor can make the procedure difficult, for example if the lesion is located near the porta hepatis, when transvenous biopsy through hepatic veins is required. Additionally, patients with focal liver lesions are usually young, and the risk of hemorrhage after biopsy should be considered. Tumor markers can be unspecific in the case of focal liver lesions. In conclusion, we propose CEUS as a promising option for monitoring the evolution of focal liver lesions due to the advantages mentioned above. Other authors report the following observations in ultrasound and CEUS of these tumors: FNH in standard grey scale ultrasound is highly nonspecific [10-12]. It can be hypoechogenic and isoechogenic compared to the liver parenchyma [3, 9]. In examinations using contrast media benign lesions enhance homogenously, which is an advantage compared to traditional ultrasonography. Characteristic features of FNH are central fibrous scar and central feeding artery (easy to detect in Doppler mode). A central fibrous scar is expected in the case of > 3 cm lesions. Specifically, in CEUS the most characteristic enhancement pattern is the “spoke wheel pattern” which is a result of the presence of a central artery feeding its branches. This pattern can also be observed in the case of HCC and cirrhosis, which requires differentiation; also it is not always present in small lesions (< 3 cm) [10, 11]. Specific arterial phase centrifugal enhancement is observed; enhancement is usually sustained in the portal phase and late venous phase. The central fibrous scar in venous phases may be hypoenhanced. In the case of HCA highly nonspecific images in grey scale ultrasound are also observed. Moreover, some hypoechogenic spaces can be noted. They result from necrosis and calcifications caused by tumor bleeding [7, 8]. High peak and low flow impedance from subcapsular arteries in color Doppler are characteristic for HCA [8]. After contrast administration centripetal enhancement is observed. In the arterial phase the lesion is completely hyperenhanced. In the portal phase the lesion can persist in hyperenhancement or become isoenhanced. From this moment of examination non-enhancement spaces can be observed. If washout or hypoenhancement occurs in the portal or late venous phase, malignant transformation can be suspected [3, 11]. In our group no lesion exhibited the mentioned features. CEUS exhibits several advantages compared to MRI/CT. The main feature of CEUS is that a low dose of contrast agent does not overload the kidneys; therefore the procedure can be offered to patients suffering from renal insufficiency. Furthermore, patients are not exposed to radiation [7]. In addition, CEUS is a cheap and readily accessible examination which does not require special preparation of the patient [3]. However, the localization of focal liver lesions should be confirmed before CEUS examination due to the limited analysis area during CEUS and the possibility of overlooking some changes in the arterial phase. In general, CEUS is more suitable for monitoring focal lesions than for making an initial diagnosis. The lesion typically appears hyperenhanced only in the arterial phase, and in later phases it becomes similar to the liver parenchyma, diminishing its diagnostic value. Consequently, an initial MR or CT examination should be performed to evaluate the entire liver accurately. Once the diagnosis is confirmed, CEUS can be considered as a promising method in sequential follow-up examinations. Our study has some limitations. There was no histopathological confirmation of tumor diagnoses, as routine biopsies were not performed due to a lack of indications. Patients with low hemodynamic efficiency might experience delayed contrast influx, which could affect the observations. The data were registered from 20 s after contrast appearance, but contrast can appear later in some cases. In our study, the enhancement values are compared with liver parenchyma, which is considered healthy. Benign liver tumors can also be observed in liver diseases such as steatosis or cirrhosis, which may complicate the assessment of enhancement values. Furthermore, the efficiency of the proposed algorithm needs confirmation in longer-term follow-up studies due to the short presence of CEUS in regular use. Personally, we are planning further research using a higher mechanical index (1.2) to obtain better quality images.
Conclusions
Compare contrast-enhanced ultrasound can be considered as a promising alternative method in diagnostics of focal liver lesions. However, MRI or CT with contrast enhancement remains the gold standard in this case, and one of them should be performed at the beginning of the diagnostic process due to complex overview of the liver parenchyma and pathological lesions. It is essential to obtain a tumor-parenchyma enhancement difference of 30 dB early in the study and to equalize tumor-parenchyma enhancement in the later stages of the observation. In conjunction with similarity of size and enhancement patterns of benign tumor lesions in MRI and CEUS confirmed in our study, we suggest that CEUS can be successfully used as a tool in monitoring FNH and HCA. When some changes in CEUS examination are observed, it gives an indication to perform MRI/CT. Therefore, we recommend CEUS in standard examination of focal liver lesions evolution.