Introduction
Ultrasound-guided fine-needle aspiration biopsy (FNAB) remains the primary diagnostic method for evaluating thyroid nodules due to its simplicity, cost-effectiveness, minimal contraindications, and low complication rate. However, its diagnostic accuracy is notably operator dependent [1]. Fine- needle aspiration biopsy can occasionally produce sparse cellular samples, leading to non-diagnostic or indeterminate results [1, 2]. Such inconclusive findings can delay the start of appropriate treatment, particularly when there is an indication of malignancy without definitive tumour characterisation. Although the American Thyroid Association (ATA) advises repeating FNAB in cases with inconclusive outcomes [3], there is a subset of patients for whom multiple FNAB attempts fail to yield a clear diagnosis. For rapidly enlarging neck masses indicative of anaplastic thyroid cancer (ATC) – a particularly aggressive type of cancer – core needle biopsy (CNB) has been suggested as a viable alternative [4]. In addition, various studies have emphasised CNB’s capability to accurately diagnose a significant portion of indeterminate thyroid lesions with a low risk of complications [5, 6].
Anaplastic thyroid cancer, although it represents only 2–5% of all thyroid tumours, is responsible for a quarter of all thyroid cancer-related deaths. In selected ATC cases where R0 or R1 resection seems likely, extensive surgery requires immediate adjuvant radiotherapy and/or chemotherapy. When surgery is not an option, primary chemoradiation, palliative radiotherapy, systemic therapy, or best supportive care are used. The prognosis is poor, with a median survival of 5 months and a 2-year overall survival of 10%. However, recent personalised molecule-based treatment strategies are achieving improved cure rates. The combination of dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) is currently recommended for patients with BRAFV600E mutation-positive ATC [7]. Core needle biopsy provides more extensive tissue samples, facilitating thorough histological, immunohistochemical, and molecular examinations.
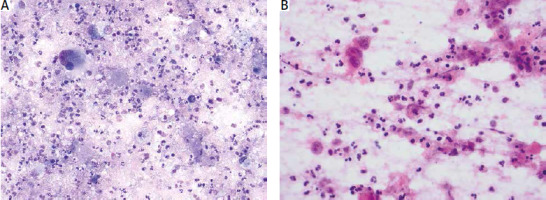
The histopathological characteristics of ATC (extensive necrosis, haemorrhage, and significant inflammatory infiltration) make it challenging to diagnose through FNAB (Fig. 1 A, B).
Fig. 1
Fine needle aspiration smears of anaplastic carcinoma. Extensive background necrosis, abscess-like inflammatory infiltrate, and tumour diathesis can obscure cancer cells, making cytological diagnosis difficult. Giemsa (A) and haematoxylin and eosin (B) stains
Given ATC’s swift progression, timely diagnosis is essential. Core needle biopsy provides more extensive tissue samples, facilitating thorough histological, immunohistochemical, and molecular examinations. This is especially important when differentiating anaplastic cancer from high-grade lymphoma, because their treatments vary widely, but certain cytological characteristics and clinical presentations of these 2 malignancies overlap. Although CNB is somewhat more invasive than FNAB, it is deemed safe when performed by experienced professionals, presenting only minor complications [8–12]. Some studies even regard CNB as superior to repeated fine needle aspiration (FNA) for ambiguous nodules [13]. This study aims to assess the efficacy and accuracy of CNB in diagnosing rapidly growing thyroid tumours clinically suspected to be ATC.
Material and methods
A retrospective assessment was conducted on 31 patients with suspected malignant, rapidly growing thyroid tumours larger than 4 cm (range: 4.3–15 cm). These patients were admitted to the Maria Skłodowska-Curie National Research Institute of Oncology in Warsaw, Poland. Between November 2020 and May 2023, all patients underwent a CNB of the thyroid gland. The decision to perform CBN was made on non-diagnostic or unsatisfactory results of repeated fine needle aspirations or, in cases of high suspicion of aggressive malignancy, a biopsy was performed simultaneously with FNAB.
The patients’ medical histories were meticulously evaluated, particularly regarding the use of antiplatelet or anti-coagulation therapies. The therapy was corrected or discontinued according to the existing recommendation [14]. The biopsy was performed under local anaesthesia using 1% lignocaine. An 18-G × 100 mm double-component, spring-activated needle guided by ultrasound was used. A 2–3-mm skin incision was made with a surgical blade. The needle was then positioned above the neck mass and inserted to deploy the cutting cannula. Typically, one or two tissue samples were secured through the same skin incision, taken from different areas within the nodule. Once retrieved, the samples were immediately fixed in a 10% formalin solution. After the procedure, the skin was disinfected, and no sutures were applied. Patients received manual neck compression and were observed in the hospital for a period ranging 20–30 minutes to an hour. Post-procedure, no antibiotics or analgesics were necessary.
Subsequently, the samples underwent microscopic exa-mination and immunohistochemical staining. Selected samples were also subjected to molecular tests using either the quantitative polymerase chain reaction method (BRAF V600 Mutations Detection Kit) or next-generation sequencing (NGS) analysis (Fusion Plex CTL Kit for Illumina). The cytopathological findings were retrospectively reviewed, and the diagnostic efficacy of CNB was assessed for all patients. The procedure’s ability to diagnose the malignancy of the lesion, particularly its histotype, and obtain ample material for molecular analysis was scrutinised.
Results
The analysed group consisted of 31 patients: 15 males and 16 females. The mean age was 68.9 years, with range 42.3–87.1 years. Core needle biopsy provided a sufficient tumour sample for accurate diagnosis in 29 out of the 31 patients (93.5%).
In 17 patients, CNB was performed after one or two nondiagnostic or unsatisfactory fine needle biopsies, or the diagnosis was insufficient to decide on further management. In 11 cases, fine needle and CNB were performed simultaneously. In 3 patients, only CNB was performed.
An assessment of CNB complications revealed minor local bleeding in 2 patients and neoplastic cell dissemination along the needle tract in one case. Other than mild local pain, no additional side effects were reported within the analysed group.
In 29 diagnostic cases, additional immunohistochemi-stry (IHC) staining was performed after the microscopic evaluation of specimens routinely stained with haematoxylin and eosin to establish the most precise diagnosis. In 2 cases, the collected material was non-diagnostic; one sample contained only hyaline stroma, and the other contained necrotic tumour tissue. Most often (in 19 cases), a panel of antibodies was used: PAX8, CKAE1/3, LCA, p40, CK19, TG, TTF-1, CyclinD1, BRAF, and Ki-67. In the remaining 10 cases, diagnostics were extended depending on the microscopic image with additional selected antibodies: Tg, calcitonin, chromogranin, SOX10, CD31, CD30, CD34, CD20, S100, CK7, CK20, NUT1, GCDFP-15, desmin, p53, or INSM1.
Genetic tests were performed on 14 patients. Positive results were obtained in 6 cases: in 2 anaplastic carcinomas, the molecular test showed a BRAF mutation (V600), while the NGS method detected a pathogenic variant in the NRAS gene p.(Gln61Lys): c.181C > A in one case of anaplastic carcinoma and a pathogenic variant in the HRAS gene p.(Gln61Arg):182 A > G in one case of poorly differentiated carcinoma. Additionally, MET fusion (MET; ST7[1]-MET[2]) was found in one anaplastic carcinoma, and NSD3-NUT M1 fusion was detected in one case of NUT carcinoma (Table 1).
Table 1
Histopathological diagnoses in the analysed group
| Diagnosis | Numbers | Genetic change |
|---|---|---|
| Anaplastic thyroid carcinoma | 14 | BRAF (V600) mutation (2 cases) NRAS p. (Gln61Lys); c181C > A (1 case) MET fusion ST7[1]-MET [2] (1 case) |
| Poorly differentiated carcinoma | 3 | HRAS p.(Gln61LysArg):c182A > G |
| Pleomorphic sarcoma | 2 | – |
| B-cell lymphoma | 2 | – |
| NUT midline carcinoma | 1 | NDS3-NUTM1 fusion |
| Metastatic carcinoma | 6 | – |
| Squamous cell carcinoma (5 cases) | ||
| Pulmonary adenocarcinoma (1 case) | ||
| Benign lesion | 1 | – |
| Non-diagnostic | 2 | – |
Discussion
Promptly obtaining an accurate diagnosis for large and rapidly growing neck tumours is crucial. Such tumours often present life-threatening risks, whether through mechanical compression or infiltration of essential neck structures. Anaplastic thyroid cancer is the most commonly observed neck tumour showing such clinical progression. Besides its dangerous local symptoms, ATC has a high propensity for distant metastases, leading to rapidly fatal outcomes. The disease-specific mortality rate is reported as over 68% at 6 months and about 80% at 12 months. The median overall survival (reaching 3–10 months) is extremely poor because of the concurrent resistance to radiotherapy and systemic chemotherapy [15]. Conversely, many large thyroid masses that are not ATC, such as lymphomas, can be effectively addressed with specific treatments, avoiding surgery. Both thyroid lymphoma and ATC have similar clinical presentation, usually a rapidly enlarging lump in the neck, neck pain, dysphagia, hoarseness, and stridor. However, the prognosis and treatment vary widely. Therefore, a precise identification of their histological type is essential to determine the best therapeutic approach before the condition becomes life-threatening. Although FNAB plays an essential diagnostic role in the initial assessment of ATC, supplementary techniques and molecular analysis are often required for a definitive diagnosis. The sensitivity of FNA cytology in diagnosing ATC is relatively low, reported between 50 and 61% [16, 17]. An accurate diagnosis in over 60% of cases necessitates IHC on a cell block procured from a cell-rich aspirate [18]. Modern diagnostic techniques, including genetic and immunohistochemical markers, enable the implementation of targeted treatments, thus improving survival rates in individual cases. In recent years, a growing number of clinical trials have confirmed that molecular profiling of tumours is fundamental in ATC for predictive, prognostic, as well as therapeutic reasons. Molecular analysis in individual cases is crucial for implementing new treatment strategies. Targeted the-rapies include tyrosine kinase inhibitors (pazopanib), multikinase inhibitors (sorafenib, sunitinib, lenvatinib), and selective BRAF kinase inhibitors (vemurafenib, dabrafenib, encorafenib), often combined with MEK inhibitors (trametinib, cobimetinib, binimetinib or selumetinib). A variety of simple and combined targeted therapies have already been approved, showing promising results in some studies. Despite checking for the presence of BRAF V600E mutation, analysing microsatellite instability (MSI) status is strongly recommended because a small subgroup (7.4%) exhibits MSI, and up to 10–15% demonstrate acquired mutations in the DNA mismatch repair pathway. These patients may benefit from recently approved targeted therapies, which are currently being evaluated in numerous clinical trials [15].
As the basic framework of diagnostics, the recently updated ATA guidelines suggest considering repeated FNAB or molecular testing for thyroid lesions with inconclusive biopsy results [3]. The literature indicates that histological examination of tissue samples from CNB can provide clarity for many ambiguous thyroid nodules [5]. For thyroid nodules with previously inconclusive FNAB outcomes, several strategies are proposed, including repeated FNAB [19], molecular testing [20–22], diagnostic surgery [2, 23], or CNB [5, 6]. Some recent studies have underscored CNB’s potential to accurately diagnose a considerable number of indeterminate thyroid nodules [5, 24] with a minimal rate of complications [25]. However, challenges persist when results from repeated biopsies remain unclear. Immunohistochemical examinations do not always enhance diagnostic accuracy because, in some cases, ATC shows negative expression of most of the antibodies used in the diagnosis of this neoplasm. While diagnostic surgery yields a definitive diagnosis, it carries complication risks with the added concern that surgical resection might be unwarranted if a benign outcome is attained.
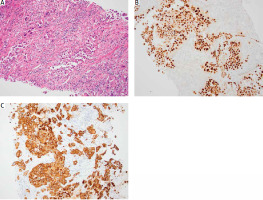
Recent trends show that certain centres increasingly recognise CNB as a safe, tolerable, and effective diagnostic tool for indeterminate thyroid nodules [5, 6, 8, 9, 26]. Some studies found no added benefit of CNB over FNAB [27–31], but others have reported CNB to be more accurate [27, 32], and particularly more decisive than repeated FNAB for initially diagnosed ambiguous lesions [13]. Core needle biopsy provides ample thyroid nodule tissue, aiding in understanding architectural histological structures and further immunohistochemical stainings (Fig. 2 A–C). Some authors even suggest it as a potential first-line diagnostic alternative. Research indicates that CNB can classify up to 98% of indeterminate nodules as malignant or benign [8], boasting a sensitivity of 91% and a specificity of 99% [33].
Fig. 2
Section of core needle biopsy with inflammatory infiltrate and well-preserved areas of pleomorphic cancer cells (A), immunoreactivity for PAX8 and variable keratin expression are common and can be prominent in a subset of cases; PAX8 (B) and keratin (C)
In our studied group, CNB was applied to patients suspected of aggressive malignant neoplasms when a satisfactory diagnosis for subsequent treatment was elusive or when the clinical picture indicated a high risk of aggressive malignancy requiring quick diagnosis as a first-line method combined with FNAB. In these scenarios, the advantages of diagnosis considerably overshadowed the risks and potential complications. The acquired material facilitated the preparation of an IHC staining panel and, in some instances, was used for molecular analysis. This method is relatively straightforward, moderately priced, and usually yields satisfactory results within a few days.
Core needle biopsy has proven to be a reliable and effective diagnostic tool for indeterminate thyroid nodules, potentially circumventing unnecessary surgical interventions [5, 6]. Histological examination shows that most (70–80%) of these nodules are benign [27]. Compared to FNAB, CNB was able to detect malignancy in an additional 27% of cases [30]. Even in the absence of explicit guidelines for using CNB for indeterminate thyroid nodules, evidence underscores its efficiency in minimising inconclusive results and enhancing the diagnostic precision for nodules with initially uncertain results [34]. Hence, to reduce the probability of surgical incisional biopsy and curb treatment delays, CNB stands out as a diagnostic tool with superior sensitivity to FNAB. Given its less invasive nature compared to incisional biopsy, it offers a similar depth of IHC and molecular marker analysis.
Conclusions
Core needle biopsy serves as an effective means of obtaining surgical specimens for histopathological evaluation, presenting an alternative to surgical resection. This technique poses minimal complication risks and provides more extensive tissue samples compared to FNA, which facilitates immunohistochemical and molecular examinations increasingly used in recent personalised molecule-based treatment strategies. In situations of diagnostic uncertainty, CNB facilitates the determination of an appropriate treatment course, potentially averting unnecessary surgical removal of the thyroid. Core needle biopsy should be considered when FNAB results remain inconclusive, especially in cases of advanced thyroid tumours with suspected malignancy. For aggressive, rapidly enlarging thyroid masses, CNB could emerge as the primary diagnostic approach. By adhering to strict criteria within a hospital setting, CNBs ensure accurate diagnoses with reduced risks. However, to further establish the advantages of CNB over FNA, more comprehensive controlled studies with larger and more consistent patient groups are essential.








