Introduction
Heavily calcified vessels constitute a major obstacle for percutaneous coronary intervention (PCI), as it may be associated with stent delivery failure and suboptimal deployment. Consequently, the risk of acute procedural complications, e.g. loss of stents or perforation of the coronaries is increased. The risk of complication such as restenosis or stent thrombosis is also increased by calcifications [1]. PCI in severely calcified coronaries has been associated with higher rates of myocardial infarction (MI) and death in addition to increased frequency of coronary revascularization. As such, significant coronary artery calcification (CAC) is considered to be an independent predictor of worse prognosis post PCI [2]. The SYNTAX score, which is designed to assess the complexity of coronary artery disease and aids in choosing a revascularization method, allocates two points per lesion when there is heavy calcification present on fluoroscopy [3].
The diagnosis of moderate to severe calcification is increasing as techniques for detecting coronary calcification improve. Recent registries and meta-analyses estimate a prevalence of 18% to 26%. Patients with advanced age, chronic kidney disease, type II diabetes mellitus, systemic hypertension, and dyslipidemia have the highest incidence of vascular calcifications [4–6].
Pathophysiology of coronary artery calcification
It is now accepted that the vascular calcification process is mediated by complex and highly regulated mechanisms involving activation of genetic factors, cellular signaling pathways, as well as hormones [7]. Vascular calcification can be located in the media (within the medial smooth muscles) or in the intima (within atherosclerotic plaque).
Medial calcification typically occurs in the peripheral arteries of lower limbs, which leads to decreased elasticity and is commonly observed in patients with peripheral vascular disease. Various predisposing factors, such as hypercalcemia, hyperphosphatemia and increased parathyroid hormone, associated with renal failure and hemodialysis, exert a significant influence on the progression of medial calcification. Despite being thought to be a harmless process, medial calcification increases arterial stiffness, which elevates the risk of arterial hypertension and other adverse cardiovascular events [8].
The mechanism of intimal coronary calcification differs in that; dysmorphic calcium development is driven by chondrocyte-like cells, in a process, which is directly related to vascular inflammation. Cholesterol deposits under the endothelium initiate an intense inflammatory response that causes the development of microcalcification, while the differentiation of vascular smooth muscle cells and pericytes promote deposition of bone as part of the necrotic core of atheroma. The deficiency of calcification inhibitory factors such as osteopontin and osteoprotegerin, further impairs the balance of osteogenic and osteoclastic mechanisms [9–11].
Apoptosis of activated macrophages leads to relatively large punctate-shaped microcalcifications, while apoptotic smooth muscle cells produce fine microcalcifications. These microcalcifications are mostly found in the deeper layers of the necrotic core close to intimal elastic lamina. They coalesce with each other creating larger calcific deposits in the form of speckles and plates which may breakdown into calcified nodules.
It was observed that calcified nodules are commonly situated at sites subjected to high motion or torsion in the artery during the cardiac cycle. They are most predominant in the right coronary artery (61%) and at its mid-segment (56%) [12]. There are two types of calcified nodules based on the direction of growth; protruding nodules that bulge into the vessel lumen and are covered by a smooth and regular fibrous cap or eruptive nodules, which extend inwards through the intima and have a rough surface and an irregular cap. This may result in fibrin deposition, which can lead to acute luminal thrombosis at any time. In 2% to 7% of coronary artery thrombi [13] and 4% to 14% of carotid artery thrombi [14], calcified nodules were the underlying mechanism of the acute events. Compared to eruptive nodules, protruding nodules have better prognosis; however, they may lead to suboptimal stent expansion and reduced minimal stent area [15].
On the other hand, spotty calcifications in the fibrous cap, by creating high local stress, make the plaque unstable and may induce its rupture [16]. This vicious circle of recurrent plaque rupture with subsequent healing ends up with the creation of fibro-calcified occlusive lesions and are usually associated with chronic stable angina and sudden cardiac death [17]. Generally, fine calcifications are found in unstable plaques and are usually associated with acute coronary syndromes [18].
Diagnosis of coronary artery calcification
The assessment of CAC is essential for appropriate management of calcified coronary arteries. This can be achieved using various tools such as non-invasive coronary computerized tomography angiography, fluoroscopy during angiography, and intravascular imaging using intravascular ultrasound (IVUS) or optical coherence tomography (OCT) (Table I, Figure 1).
Table I
Comparison between different imaging modalities of calcified coronary arteries
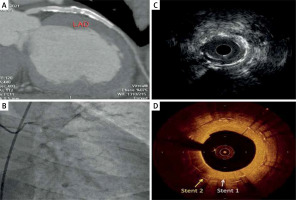
Figure 1
Imaging of two overlapping LAD stents by different imaging modalities: A – CT angiography showing patent stents, B – flouroscopy showing two LAD stents with some degree of calcification, C – IVUS image showing superficial calcifications and non-obstructive plaque ISR: however, the stent stratus is not visualized clearly, D – OCT image showing two distinct overlapping stent strata with neoatherosclerosis and calcifications. The plaque morphology can be easily inspected due to high resolution of the OCT image
Coronary computerized tomography angiography (CCTA)
CCTA has a high sensitivity and specificity to detect calcium, which makes it the only noninvasive test to quantify coronary calcifications [19]. Calcification of the coronary arteries is very specific for atherosclerosis, but it is not possible to detect or exclude the presence of obstructive coronary artery disease based solely on calcium detecting imaging. CAC scores are shown to be highly predictive of the occurrence of worse cardiac events in a number of population studies. The addition of calcium scores improved the prediction of cardiovascular events by the Framingham risk score [20–22]. A zero CAC score was found to be associated with excellent prognosis in asymptomatic and low- or intermediate-risk patients, with annual death rates less than 1% up to fifteen years. Even small amounts of CAC are associated with an increased risk.
The last ESC Guidelines for Chronic Coronary Syndrome recommended coronary CCTA as the first modality for diagnosing coronary artery disease (CAD) in symptomatic patients at low- or intermediate-risk for obstructive CAD (Class I) [23]. Recently, the ESC guidelines advise that in the assessment of the cardiovascular risk, it is possible to use CAC scores as a risk modifier (Class IIb, level of evidence B), particularly in patients at intermediate clinical risk [20]. However, the role of CAC scoring in the primary prevention of the cardiovascular risk in patients treated with lipid- or blood pressure-lowering medications remains to be demonstrated.
CAC scoring uses non-contrast ECG-gated CT to provide measures of coronary atherosclerotic burden. It is identified by any area of hyper-attenuation, defined as a minimum of 1 mm2 with > 130 HU, or by having ≥ 3 adjacent pixels using the Agatston method (Table II). The sum of all identified calcium lesions constitutes the calcium score that is used for the prediction of the cardiovascular risk for development of future clinical events [24]. Compared to fluoroscopy, CT was found to be more sensitive, detecting coronary calcium in 90% versus 52% in case of fluoroscopy. The radiation dose associated with the scan is small. CCTA is able to characterize coronary calcium plaques, specifically the identification of spotty calcification, which is a marker of vulnerable plaques.
Table II
The Agatston method for estimating CAC
Interestingly, some studies demonstrated that a zero calcium score is not a reliable marker of the absence of significant CAD. A subgroup of the CORE64 study demonstrated a NPV of 68%, concluding that a CAC score of zero cannot exclude CAD. However, it should be noted that the study population had a higher pretest probability of CAD [25]. The CONFIRM registry [26], which included over ten thousand symptomatic patients, documented coronary stenosis ≥ 50% in 3.5% of the patients with zero CAC score. Of them 1.4% had coronary stenosis ≥ 70%. Another study of 6541 low-to-intermediate ASCVD-risk patients [27], investigated the presence of obstructive CAD and high-risk plaque (HRP) criteria in patients with zero (< 1.0 AU) and ultralow (0.1–0.9 AU) calcium scores. In 1289 patients with zero CAC score, 25.9% had CAD (5.1% > 50% and 20.8% less than 50% stenosis) and 6.8% had HRP. In the ultralow CAC score group (162 patients), > 50% stenosis was found in 16.6%, and plaques with HRP-criteria were present in 19.1%. These data highlight the importance of considering the patient symptoms and pre-existing risk factors while interpreting CAC score.
Despite this, there are no clearly established criteria by which CCTA can predetermine how PCI should be performed. The calcification remodeling index is a ratio of the lumen area of the most severely calcified site to the lumen area of the proximal reference. It has been proposed to evaluate when rotational atherectomy (RA) might be required based on CT criteria. In a retrospective study, it was significantly associated with the rate of RA application to facilitate stent implantation, with an index of ≤ 0.84 independently predicting the need for RA prior to PCI [28]. This score requires further validation before it becomes clinically useful.
A recently published study showed that a mean calcium density of > 637 HU was the strongest predictor of the need for RA during PCI, among other parameters including the calcification remodeling index, calcium length, calcium arc quadrant, and calcium score [29]. Awareness of the need for potential plaque modifying tools is mandatory while managing patients with a high calcium score. A CCTA-derived 3D reconstruction of the coronary vasculature has been developed. It provides a road-map to allow a full pre-planning of coronary intervention [30]. However, CCTA cannot be used to guide PCI since it is unable to provide information about the degree of CAC modification during the procedure.
Some studies have demonstrated a positive relationship between the progression of atherosclerotic plaques, measured by CCTA as an increase in CAC score, and the future cardiovascular risk [31, 32]. However, there is no widely accepted method for calculating the progression of atherosclerotic plaques. An annual increase of ≥ 15% in the volume of coronary calcium was suggested to predict a 17-fold increase in the cardiovascular risk [33]. Hokanson et al. [34] suggested an increase in coronary calcium volume ≥ 2.5 mm3 to be a cut-off for plaque progression.
Fluoroscopy
Historically, fluoroscopy was the earliest modality to detect calcifications. In patients who have not undergone CCTA prior to angiography, it may be the first opportunity to diagnose calcified lesions. Angiographic calcifications are often classified into three categories: none/mild, moderate, and severe. CAC is regarded as moderate when there is radio-opacity observed only with the cardiac motion before contrast is injected, whilst severe calcification is radio-opacity seen irrespective to the cardiac motion, visible as a tram-track line on both sides of the arterial lumen. Angiography, however, underestimates the degree of calcification and is unable to estimate the calcium depth. Occasionally severe calcification can resemble and be mistaken for thrombus when severe calcified nodules prevent contrast penetration and appear as filling defects in the lumen. Although being highly specific in detecting CAC with a remarkably high positive predictive value, coronary angiography has low to moderate sensitivity of about 38% compared with CCTA and intravascular ultrasound [35, 36]. A more recent study showed similar accuracy for coronary angiography of about 40.2% when compared to IVUS [37]. The diagnostic accuracy increases when superficial calcification, calcium arc > 180° and length > 6 mm is present [38].
Intravascular ultrasound (IVUS)
Ultrasound has a resolution of 150–200 µm but does not penetrate calcium. The leading edge of calcification can be visualized as a bright hyperechoic deposit but behind the calcium there is a blank acoustic shadow and further details of the vessel wall are invisible. Severe calcification can result in reverberations behind the superficial plate of the calcium. IVUS quantifies CAC by measuring the extent of the arc of uninterrupted calcium (measured in degrees) as well as its length, and it is classified as superficial (close to the lumen) or deep (behind the lumen in the deep media or adventitia) where it has little impact on the lumen. The most relevant calcium is superficial calcium in an arc of more than 180° [37, 38]. The longer the arc extent in degrees and length in millimeters, the higher the incidence of stent underexpansion.
Furthermore, IVUS has demonstrated a great value in guiding PCI for all lesion subtypes, including calcified ones. A recent meta-analysis of 27,610 patients showed that IVUS-guided PCI patients experienced a relative risk reduction of 33% of cardiovascular death as compared with ordinary angiography-guided PCI. The superiority of IVUS to characterize atherosclerotic and calcification burden, calculate the proper stent size, and avoid underexpansion or malposition, explains these results [39]. Finally, IVUS may also assist in predicting and identifying procedural complications such as dissection or intramural hematoma.
Limitations of IVUS include inability of the catheter to cross highly calcified lesions. IVUS is also unable to measure calcium depth or detect microcalcifications (< 50 µm) due to being below the spatial resolution threshold. IVUS failed to detect calcium in 14.8% of plaques containing histopathologic calcium in an in vitro study investigating IVUS imaging of human coronary artery explants. Deep calcium masked behind the vast necrotic cores that produce echo attenuation is the most probable explanation for these results, along with microcalcification [40]. The same principle applies to calcified in-stent restenosis where IVUS fails to penetrate multiple layers of stent struts. A recent study showed promising results of IVUS ability to estimate calcium depth. A smooth calcium border with reverberation artifact was associated with a calcium thickness < 0.5 mm in 54.6% of cases. On the other hand, an irregular border without reverberation artifact within the region of signal-drop-out was associated with a thickness ≥ 0.5 mm in 75.9% of cases [37].
Optical coherence tomography (OCT)
OCT has a resolution of 10–20 µm. It measures light backscatter and calcium is seen as a low signal area with well-delineated sharp borders. Because, unlike ultrasound, light is able to penetrate calcium easily, OCT measures calcium volume, area and depth in addition to calcium arc and length. It can also detect microcalcifications [41]. Currently, it is the most accurate technique to estimate calcium volume, derived from its unique ability in measuring calcium thickness and longitudinal extension [42].
Calcium detected by OCT may be misinterpreted as necrotic core or lipids. It should be noted that the signal-poor regions of lipid or a necrotic core have poorly defined or diffuse borders while the signal-poor regions of calcium are sharply delineated. Although there is limited information on the various patterns of CAC, it seems that plates of calcifications would most likely be observed by this technology. Nodal calcification appears very differently from calcified plates, showing a variety of signals with the highest attenuation likely to be caused by fibrin interposed in these nodules [43]. OCT has the ability to differentiate between eruptive and non-eruptive nodules. In the latter, the lumen surface appears to be irregular [40].
Due to its higher spatial resolution, OCT was found to be superior to other imaging modalities in guiding the PCI procedure. It can accurately detect the reduction in calcium volume, increase in lumen gain and occurrence of fractures after application of calcium-modifying techniques [44].
One of the major limitations of OCT is the need for complete blood clearance of the lumen. This leads to suboptimal image quality in case of very large caliber or ectatic vessels as well as ostial LM or RCA lesions, where lumen clearance is difficult to achieve. Another limitation is the need for careful interpretation of the generated images due to suboptimal software tracing of the true lumen due to the presence of thrombi or multiple layers of stents. Furthermore, the identification of CAC is an operator-dependent process and it can be easily misinterpreted as necrotic core by inexperienced operators. Recently, a fully automated artificial intelligence (AI)-based software has been introduced to avoid operator’s interpretation bias with an accuracy up to 88.5% in detecting CAC [45].
A novel hybrid IVUS-OCT system was found to be non-inferior to each technique when performed separately. The PANOVISON trial [46] proved the feasibility and safety of the hybrid system in 100 patients undergoing PCI. Moreover, the higher pullback speed of this new technology permitted a shorter imaging time with a longer pullback length. Since all hybrid imaging was acquired after stent implantation, the hybrid catheter crossability through culprit lesions as well as culprit plaque composition could not be assessed adequately.
Assessment of the degree of calcification
In order to effectively grade calcium as mild, moderate or severe, the following measurements need to be acquired: the calcium arc, the length of the calcified segment and the thickness of the calcium. A scoring system for coronary calcification have been proposed by Fujino et al. [47]. With both IVUS and OCT, characteristics of mild to moderate calcification include an arc of calcium < 270° and length of the calcified lesion < 5 mm. Characteristics of severe calcification include an arc of calcium > 270° and length of the calcified lesion > 5 mm. Due to the ability of OCT to penetrate the calcified plaques, calcium depth can be measured, and calcium thickness of ≥ 0.5 mm is considered a marker of severe calcification.
In the event that the IVUS or OCT catheter cannot cross the lesion, it can be assumed that calcification is severe. Calcified nodules were found not to be significant by multi-variate analysis in the study conducted by Fujino et al. [47]. However, recent trials demonstrated that the presence of calcified nodules predicted long-term target lesion revascularization (TLR) and late stent underexpansion, especially within the first year, with higher rates of majopr adverse cardiovascular events (MACE) [48, 49]. Another study showed that compared to non-eruptive nodules, nodules of eruptive morphology by OCT were associated with worse post-PCI long-term clinical outcomes, despite better acute stent expansion [15]. Moreover, calcified nodules necessitate the application of debulking strategies used for severe calcification. Based on these facts, we modified the proposed OCT-based calcium score for predicting stent underexpansion. However further studies are required for validation (Table III).
Table III
Intravascular imaging-based score for CAC (IVUS/OCT)
Management of calcified coronary lesions
In the presence of moderate or severe calcification, it is recommended to shift to the use of calcium-modifying techniques. It can grossly be divided into balloon-based techniques including super high-pressure, cutting, scoring, chocolate and lithotripsy balloons (Table IV, Figure 2) as well as debulking techniques including different atherectomy devices (rotational, orbital, directional), and excimer laser (Table V, Figure 3). Finally, if all these maneuvers failed, the balloon-assisted subintimal entry (BASE) technique is recommended.
Table IV
Different types of calcium-modifying dedicated balloons
Table V
Different types of calcium-modifying debulking devices
Figure 2
Different types of calcium-modifying balloons: A – OPN NC super high-pressure balloon (OPN NC, SIS Medical AG, Winterthur, Switzerland), B – WOLVERINE Coronary Cutting Balloon (Boston Scientific, Massachusetts, USA), C – Scoreflex Scoring balloon (OrbusNeich, Hong Kong, China), D – Chocolate™* PTA balloon (Medtronic, Minnesota, USA). Reproduced with permission from each company
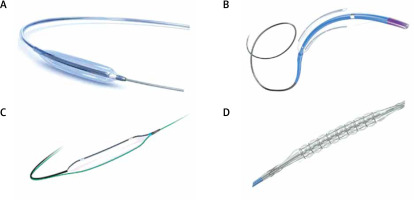
Figure 3
Different types of atherectomy devices: A – ROTAPRO Atherectomy System burr (Boston Scientific, Massachusetts, USA), B – Diamondback 360™ Orbital Atherectomy System (Abbot, Illinois, USA), Classic Crown mounted concentrically (thick single arrow) and the newer edition MicroCrown mounted eccentrically (two smaller arrows), C – HawkOne™ Directional Atherectomy System (Medtronic, Minnesota, USA), D – CVX-300 Excimer laser system (Philips, Amsterdam, Netherlands). Reproduced with permission from each company
High- and very high-pressure non-compliant balloons
The OPN NC balloon is intended to allow for a very strong pressure inside the balloon, with little change in diameter. This is achieved by the unique twin-layer, balloon-in-balloon technology, which allows for very high pressure while ensuring its uniform distribution to prevent any possible vascular damage. The ISAR-CALC randomized trial compared OPN NC balloon to scoring balloons in predilatation of heavily calcified coronary lesions. It showed comparable stent expansion (evaluated by OCT) and a trend towards improved angiographic performance of the ultra-high pressure balloon [50]. In clinical experience, compared to conventional NC balloons, there is a reported success rate of more than 90% for undilatable lesions, with a rate of less than 1% of coronary rupture [51]. The balloon has been tested up to 55 Atm without rupture; however, it has a high crossing profile making it difficult to reuse after inflation. Despite that, its profile is still better than cutting and scoring balloons [51]. The main indications for use include in-stent restenosis, calcified lesions, and undilatable lesions [51, 52].
Cutting balloons
Cutting balloons have metal atherotomes or microblades on its surface, designed to make radial incisions in the media. They therefore reduce elastic recoil, minimize neointimal proliferation and prevent slippage of the balloon during inflation, which is specifically useful for in-stent restenosis caused by intimal hyperplasia [53]. The earliest clinical applications proved to be superior to traditional balloon angioplasty, especially in terms of achieving greater luminal enlargement. Subsequent randomized data comparing conventional balloon to cutting balloon angioplasty for de novo non-dilatable lesions showed similar procedural success rates and 6-month restenosis rates (30.5% vs. 31.6%, respectively; p = 0.76), at the expense of greater perforation rates in the cutting balloon group (0.9% vs. 0%; p = 0.028) [54]. Moreover, the microblades are arranged longitudinally and in parallel along its surface, worsening balloon stiffness and crossing profile. These data have limited its use in resistant lesions. The Flextome Cutting Balloon, introduced in 1991, is available in over-the-wire or monorail wiring, with the latest version (Wolverine) has more flexibility and better crossing profile [55].
Scoring balloons
The scoring balloon is a semi-compliant balloon encompassed by nitinol spiral coils, which serve as anchors when dilating calcified or fibrotic lesions, decreasing balloon slippage, and allowing for focal, concentrated pressure during expansion to disrupt plaque integrity. They are similar to cutting balloons, but have lower crossing profiles and greater flexibility allowing softer delivery. Moreover, they can be fully expanded at relatively lower inflation pressure therefore lowering the risk of perforation and dissections [56].
There are many types of scoring balloons available. The AngioSculptTM (Philips, Amsterdam, The Netherlands) is a semi-compliant balloon with three spiral rectangular nitinol scoring elements. It is available also in a drug-coated version (AngioSculpt X) [57, 58]. A very high procedural success and target lesion revascularisation rate of 10% at 6 months was shown in a feasibility trial to treat de novo lesions prior to bare-metal stent implantation [59]. In an observational study, these results were confirmed in 37 patients who had been treated with AngioSculpt prior to the implantation of a stent against 145 patients receiving direct stenting and 117 patients having traditional plain old balloon angioplasty before a stent was implanted. IVUS assessment revealed better stent deployment in the AngioSculpt group than in the other two groups (89% vs. 74% of vessels, respectively, with an area of more than 5.0 mm2) [60].
NSE Alpha (B Braun) scoring balloon has also three nitinol wires, however, they are attached only at the proximal and distal edges of the balloon. Promising results have been obtained for the predilatation of severe calcified lesions, making it a potential choice for management of resistant calcified lesions [61].
Scoreflex (OrbusNeich) is a semi-compliant balloon that has two fixed nitinol wires on opposite sides of the balloon’s surface [62]. A case series has been described by Otsuka et al., in which a prolonged inflation of Scoreflex balloon at lower pressures was able to allow sufficient dilatation of severely calcified lesions [63].
Chocolate balloon
The Chocolate™* PTA balloon was first inttroduced to manage peripheral arterial disease as a primary (without stenting) or adjunctive therapy with a high success rate and favourable safety profile [64]. It is a semi-compliant balloon, constricted by a cage of nitinol wires. When inflated, the cage causes the balloon to form runs of segmented pillows and grooves along the entire lesion. The pillows apply force to create small dissections within the plaque and the grooves are designed to relieve wall tension and stop dissections from propagating. It is the only nitinol-caged balloon with controlled dilatation, designed for 1 : 1 vessel sizing to minimise flow-limiting dissections and reduce recoil.
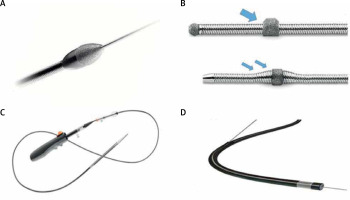
Intravascular coronary lithotripsy (IVL)
IVL uses a conventional balloon catheter with two emitters mounted inside the balloon. It emits 10 pulses in sequence at a frequency of 1 pulse/sec for a maximum of 80 pulses per catheter. The electrical discharge from the emitters vaporizes the fluid within the balloon and creates a rapidly expanding and collapsing bubble that generates pulsatile sonic pressure waves. These waves penetrate through soft tissue and cause significant shear force to preferentially fracture intimal and medial calcium within the vessel wall. Besides its remarkable high success and low complication rates, its preparation is similar to conventional balloons with a relatively short learning curve.
Kovach et al. used OCT in 31 patients with severe calcified coronary lesions who were receiving IVL. In this study, following IVL application, 43% of patients experienced lumen enlargement and one or more calcium fractures [65]. Sixty patients with severely calcified lesions (32 mm in length) in a native coronary artery were enrolled in the DISRUPT CAD I which was a single-arm study. A 95% clinical success rate was achieved (defined as residual stenosis less than 30%). The cumulative MACE rate was 5% after 30 days. Furthermore, at 30 days, no target vessel revascularization, myocardial infarction, or cardiac death occurred. There were no complications during the procedure. Acute gains in minimal luminal area were on average 3.7 mm in size in a substudy with OCT involving 31 patients [66].
The DISRUPT CAD II [67] included 120 patients with severe calcification with 100% IVL success rate. 5.8% of patients experienced the in-hospital MACE composite primary endpoint (death, TLR and MI). No procedural complications were observed. Calcium fractures were achieved in 78.7% of the 47 patients who underwent post-PCI OCT, with an average of 3.3 ±2.5 fractures per lesion. These results were replicated by the DISRUPT CAD III [68] and IV [69] trials with a sample size of 431 and 72 patients, respectively. More than 92% of cases met the critical safety endpoint of being MACE-free after 30 days. A procedural success rate of 92.4% was attained. OCT analysis revealed multiplane and longitudinal calcium fractures in 67.4% of the plaques.
In the event of a stent that cannot be expanded following deployment despite high pressure post-dilation, IVL has been reported to be safe and effective by the SMILE registry [70]. The recently published CRUNCH registry demonstrated the ability of IVL to improve stent expansion in previously underexpanded stents [71]. Currently, IVL use for stent underexpansion is off-label.
Rotational atherectomy (RA)
RA was developed in the pre-stent era to improve angioplasty results for difficult-to-dilate, calcified lesions. David Auth first proposed a rotational debulking device in the 1980s, eventually resulting in Fourier et al. performing the first in-human RA in 1988 [72–74]. In 2018, the ROTAXUS trial showed better acute lumen gain in favor of RA. However, nine-month late lumen loss was significantly higher with RA compared with stenting alone [75]. In the PREPARE-CALC trial, RA was superior to balloon-based techniques in achieving successful stent delivery, expansion with higher procedural success (98% vs. 81%). However, there were no significant differences in late complications, including TLR, stent thrombosis and 9 month late loss [76]. The problem with all the trials of rotational atherectomy is that the patients who most need RA are not eligible for any alternative treatment option and cannot therefore be randomized.
RA demonstrated great success in improving procedural effectiveness, but this has not translated into a consistent long-term benefit for restenosis and MACE [77]. Consequently, RA was officially recommended for the preparation of severely calcified balloon uncrossable or undilatable lesions (Class I, level of evidence C). The 2014 EACTS/ESC guidelines for Myocardial Revascularization recommend RA for heavily calcified or fibrotic lesions (Class IIa, level of evidence C). The 2018 Guidelines mention that RA may be applied in certain lesions, especially, those with heavy calcification, for adequate predilatation of lesions before stent implantation [78].
Regarding PCI in vessels with chronic total occlusion (CTO), randomized trials showed no benefit in using RA as the first-line strategy, but as a second-line method if the conventional techniques are unsuccessful [79, 80]. In contemporary practice, RA has been shown to perform equally well in bifurcation lesions as in non-bifurcations in terms of safety and efficacy. RA may facilitate primary side branch wiring when difficulty is encountered because of a heavy plaque burden in the main vessel preventing the crossing of a guidewire into the side branch.
The Rotablator System is composed of an elliptical burr plated with nickel and coated with diamond microscopic crystals. The burr is attached to an advancer to deliver the rotational speed. It is advanced over a 0.009-inch wire (RotaWire Floppy or RotaWire Extra Support). Conventional guide wires are not compatible with the RA (larger in diameter and their coatings may be stripped off during rotation) [74]. The burr selectively ablates the hard calcified atherosclerotic plaque while not injuring the nearby soft elastic coronary wall (differential cutting) [81].
Adequate ablation is achieved with a burr : artery ratio of less than 0.7. With more aggressive debulking (a burr : artery ratio > 0.7) there was no benefit over PTCA [82] and it was also associated with more angiographic complications [83]. A repetitive pecking motion of the burr into the proximal portion of the plaque to avoid large decelerations have yielded the most favourable outcomes [83]. Pecking motion is a quick push-forward/pull-back movement of the Rotablator burr. This motion pattern of the burr allows for frequent intermittent blood flow through the ablated segment, improving wash-out of the debris and potentially reducing heat generation and vessel spasm. Continuous saline infusion (usually mixed with verapamil or nitroglycerine) is used to reduce heat, friction, and spasm. Furthermore, RotaGlide Lubricant (Boston Scientific, Marlborough, MA, USA), which is a lipid-based specialized solution, can be added to facilitate burr and stent advancement safely, decrease the overall force needed to control the system motion and finally, to reduce the generated friction and heat [84]. The presence of olive oil, phospholipids and egg yolk among its ingredients is responsible for its remarkable lubricity [85]. However, recent studies showed that using heparin-based continuous flushing solely or mixed with other vasoactive substances was non-inferior to the Rotaglide solution [86–88].
In 2018, an upgraded system called the Rotapro was released. The Rotapro system incorporates burr controls into the advancer thus removing the need for the foot pedal. It operates with a single hybrid cable and a new console with digital display.
Earlier RA versions had larger burr sizes, which necessitate the use of femoral access. Currently, radial access can be used safely for burr sizes up to 1.75 mm, compatible with 6 Fr guide catheters. For burr sizes 2.00–2.15 mm requiring 7 Fr guide catheters, sheathless catheters enable radial access use.
RA is associated with the same spectrum of clinical complications as in PCI, including in-hospital deaths (0.6%), tamponade (0.64%), and emergent surgery (0.18%) [89]. Other complications include acute vessel closure, dissection, side branch loss, perforation, vasospasm, no reflow and burr entrapment. Slow flow or no flow occurs secondary to distal embolisation of atherosclerotic debris. It is commonly prevented with adequate antiplatelet therapy and continuous flushing of a drug cocktail containing heparin, isosorbide dinitrate and verapamil. Burr entrapment occurs with aggressive advancement of larger burrs through very eccentric and extremely calcified lesions. The absence of diamond chips on the burr’s back surface makes it hard to retrieve the burr if it passes distally to an incompletely ablated lesion. Perforation risk is greatest with a larger burr : artery ratio, eccentric lesions and in angulated segments.
Orbital atherectomy (OA)
With a stent delivery success rate of 98% and complication rate of less than 1%, the Diamondback 360 Coronary Orbital Atherectomy System (Cardiovascular Systems Inc., St. Paul, MN, USA) has been demonstrated to be both effective and safe. It received the US Food and Drug Administration (FDA) approval in 2013 to treat severe de novo calcified coronary lesions. This tool differentially sands the hard portion of the stenosis while sparing the soft tissue component by creating centrifugal force using the elliptical motion of an eccentrically mounted, single-sized crown. Pulsatile forces may also have an indirect impact on deeper calcification [89]. A larger orbit results from spinning at faster speeds because centrifugal force depends on both the mass of the crown and its rotational speed. As a result, a given crown can produce a larger lumen by simply rotating it more rapidly. The luminal gain increases with longer ablation times, more passes, or faster rotation. The elliptical orbit reduces thermal injury and slow flow by allowing blood and debris to flow freely around the crown, flushing the vessel continuously, and cooling the crown [90]. OA produces debris with an average size of 2.04 µm, 98.3% of which are shorter than red blood cells’ caliber, and 99.2% of which are narrower than capillary diameter [91].
The OA system includes a dedicated 0.012 inch stainless steel guide wire (ViperWire) and a Viper Slide which is a combination of anticoagulation and vasodilator drugs that helps to lubricate and flush coronaries during sanding. The coronary classic crown is 1.25 mm in diameter. The front and back surfaces of the crown are coated in micro-diamonds, allowing atherectomy in both forward and reverse directions, thereby increasing sanding while preventing entrapment. The OA system is compatible with 6F guiding catheters. Continuous crown movement is required to prevent uneven treatment and maintain safety. A single run should not last longer than 30 s, and the entire treatment should not last longer than 5 min [92].
In fifty patients with de-novo calcified lesions, the original ORBIT I trial demonstrated OA safety and efficacy in modifying calcified lesions to facilitate stent placement [93]. The ORBIT II trial [94] enrolled 443 patients with heavily calcified de-novo coronary lesions. Stents were successfully delivered in 97.7% of patients. Real-world data for 458 people treated with OA in three US hospitals were reported by Lee et al., confirmed positive outcomes after a year. OA was safe with a 30-day freedom from MACE of 89.6% [95] A low TLR following drug-eluting stent (DES) implantation was maintained up to 3 years. The Coronary Orbital Atherectomy System Study (COAST) assessed the latest upgrade of the OA system, the Diamondback 360 Micro Crown. This newer version has a new diamond-coated tip designed to better reach target lesions, with a 1.25 mm eccentric crown allowing for rotation at slower speeds (50,000 to 70,000 rpm) while still creating an orbit that is equivalent to that of the OA Classic Crown. To improve lesion crossability, the front edge of the crown is tapered. The study showed that 85% of patients were MACE-free at 30 days [96].
The currently recruiting ECLIPSE trial is enrolling patients with severely calcified lesions, comparing OA preparation to conventional PCI in terms of stent expansion and revascularization failure. The trial is the largest randomized coronary atherectomy trial to date, incorporating post-procedural OCT evaluation. An ongoing US observational study assessing the Feasibility of Orbital Atherectomy System in Calcified Bifurcation Lesion (ORBID-OA) will evaluate thirty patients. While the initial results have all been positive, the Manufacturer and User Facility Device Experience (MAUDE) database showed that in real-world practice there were a number of perforations related to excessive straightening of the ViperWire [97]. OA is not recommended in case of ISR, bypass grafts, presence of major dissections and thrombotic lesions. OA should be used with caution in aorto-ostial lesions as there is a significant transition from a large to small lumen. This is because OA has a less concentric crown rotation, compared to RA, which may cause dissection [98].
Directional atherectomy (DCA)
In 1990, the FDA authorized the use of directional coronary atherectomy (DCA). The device is composed of a catheter equipped with a rotating cutter that ablates plaques through a small window with the help of an inflated balloon. The rotating cutter is advanced distally, ablating the lesion and aspirating the debris. DCA is capable of debulking lesions with mixed morphologies. However, the encouraging results of many single-center experiences [99] were not reproduced in the context of randomized studies [100]. The technique is very operator-dependent and the amount of tissue disposal differs based on the the operator’s commitment to perform extensive debulking. Additionally, the DESIRE [100] and AMIGO [101] trial findings and the superior anti-restenotic efficacy of DES limited the current role of directional atherectomy. DCA is not commercially available any more for use in the coronary arteries in the USA or Europe; instead, it can only be used to treat peripheral artery disease.
Excimer laser coronary atherectomy (ELCA)
Calcified plaques are altered using photochemical (disrupting molecular bonds), photothermal and photomechanical ablation during excimer laser coronary atherectomy (ELCA). The CVX 300 System (Philips) limits medial and adventitia damage by emitting light in the ultraviolet B spectrum (308 nm) with a penetration depth of 30–50 µm using xenon chloride. The released microparticles are less than 10 µm in size and consequently have a low effect on the microcirculation [102]. ELCA catheters should be selected based on a catheter : vessel diameter ratio of 0.5 : 0.6. It is compatible with 0.014 inch guidewire. During its technical development, it was discovered that ultraviolet laser energy is significantly absorbed in contrast medium or blood, causing significant acoustic effects that produce fast-expanding and imploding vapour bubbles that quickly dilate the nearby arterial segment for a few microseconds before invaginating it. This can cause tissue disruption, leading to dissections or even vessel perforations, along with extensive medial necrosis, intramural hemorrhage, and subluminal infiltration by leucocytes [103]. Because of this, ELCA should only be carried out with continuous saline flushing during laser activation to displace blood or contrast medium from the coronary artery. Furthermore, laser-induced pathological tissue injury is minimized by using pulsed energy delivery.
Ninty-three percent of complex coronary artery lesions treated with ELCA procedures were successful, and both minor and major periprocedural complications were reported [104, 105]. Two randomized trials were conducted to compare ELCA with conventional PCI. In the multicenter AMRO study [106], 308 cases with stable angina were discovered to have no major variations in angiographic success (79% vs. 80%), Despite that, there were ten-fold higher rates of periprocedural transient vessel occlusion (0.7% vs. 7%) with higher rates of restenosis (41.2% vs. 51.5%) in the ELCA group after 6 months. There was no difference in angiographic net lumen gain (0.41 mm vs. 0.48 mm). Patients with multiple consecutive lesions, lesions in vessels smaller than 2.5 mm, or lesion location in the left circumflex coronary artery appear to have significantly poorer outcomes with ELCA [107]. The ERBAC study [108] was originally a three-arm trial comparing conventional PCI to the use of ELCA or RA in 685 patients with stable angina. Procedural success rates were comparable, with 77% and 80%, between the ELCA and PTCA groups as well as in-hospital complication rates of 4.3% and 3.1%. The periprocedural cross-over rate was almost three times higher with ELCA (5.0% vs. 15.1%). At 6-month follow-up, TLR rate was remarkably higher in the ELCA group compared to the PTCA group (46.0% vs. 31.9%). In a large registry of 9,222 patients with different percutaneous coronary interventional techniques, including 500 patients with laser angioplasty and 4,104 patients with PCI, a remarkably greater likelihood of developing restenosis with an odds ratio of 1.55 was documented [109].
Since these findings failed to support the wide appplication of ELCA and in parallel, coronary stents showed better angiographic, acute, and long-term clinical results compared to PCI, the application of ELCA was restricted to specific subgroups of lesions as a bail-out strategy, when lesions are uncrossable or undilatable for dedicated balloons or for the ViperWire or RotaWire. Currently, ELCA use is restricted to a limited number of centers.
Combination therapy
Over the last decade, marvelous advances in imaging modalities for calcifications enabled better understanding of calcium burden and distribution. In order to achieve the best stent expansion, operators frequently used a combination of two or more modalities, especially when managing uncrossable or heavily calcified thick lesions. For example, ELCA can be used in conjunction with RA (the RASER technique) [110]. The primary use of this approach is in the management of severe in-stent restenosis as it showed encouraging results in a recently published OCT study. Simultaneous contrast injection was found to potentiate the photochemical effects of ELCA on underexpanded stents [111]. However, contrast should not used in de-novo lesions because of the high risk of perforation. The ablative effects of ELCA on calcium are minimal and success relies on the ablation of more pliable tissue within the calcified lesion. It is possible, in many such cases, to create a channel using ECLA to allow microcatheter or RotaWire passage in order to facilitate RA [110, 112, 113].
A hybrid approach between rotational atherectomy and IVL (Rota-Tripsy) can also be performed. This technique would be ideal in large caliber vessels with deep calcification and critical stenosis not allowing balloon passage. This would involve performing RA with a small burr size (1.25 mm) to create a channel to allow an IVL catheter balloon to be passed through the calcified lesion. IVL would then be performed with the aim of optimal modification of deep and/or thick calcium prior to stent implantation.
While specialized balloons may have a role in mild to moderate calcified coronary lesions, they are less effective than atherectomy or lithotripsy and should be used as adjuncts to these strategies when dealing with moderate to severe calcified lesions. Amemiya et al. reported good results in terms of stent expansion when combining both RA and cutting balloons. This was a single-center observation study, using OCT to assess the number and thickness of calcium fractures and the degree of stent expansion [114].
Balloon assisted subintimal entry technique (BASE)
In the circumstances of failure of all the previously mentioned tools, or in case of wire uncrossable lesions (i.e. CTO), the BASE technique can be used as a last resort. It depends on extraplaque subintimal lesion crossing. A limited dissection is created by inflating a slightly oversized balloon (1.2 : 1 vessel reference size) proximal to the uncrossable lesion, followed by the introduction of a polymer jacketed wire into the subintimal space to bypass the lesion followed by re-entry to distal true lumen. Subsequently, a balloon is advanced next to the lesion over the extraplaque guidewire and inflated to 8–10 atm to crush the lesion externally. A modification of this technique was by the addition of IVL [115]. This is a complex maneuver that requires experience in CTO dissection and re-entry techniques. Furthermore, there is a higher risk of perforation and extraplaque hematoma formation [116].
Algorithm for management of coronary calcifications
A clear indication for PCI should be established as the first step. In case of borderline or unclear lesions, invasive functional assessment of lesion severity should be performed. In case of calcified lesions, intravascular imaging is essential for the assessment of the degree of calcifications. Lesions with moderate to severe calcifications will probably require the use of calcium-modifying tools. Before starting PCI, operators should put a plan including patient tolerability to the intervention, availability of hemodynamic support or urgent bypass surgery. Lesion and vessel characteristics should be evaluated to determine the most appropriate calcium-modifying tool. The vessel reference size, minimal luminal area, lesion length, eccentricity, tortuosity, angulation and relation to large side branches as well as nodularity of calcium should be taken into consideration. Before starting, the operator should check for the required materials (i.e. wires, special balloon sizes). A proposed algorithm based on the latest updates in management of calcified coronary lesions is summarized (Figure 4).
Conclusions
Despite the current marvelous advances in medical technology, calcified coronary lesions remain a persistent challenge. With the advent of novel multimodality imaging technologies, our understanding of the different types and distribution patterns of calcifications improved considerably. None of the available calcium-modification techniques is able to address all the different patterns of coronary calcification. Incorporation of intravascular imaging is essential for individualizing the best calcium-modifying approach for each lesion and guiding final stent optimization. Operators should be familiar with the novel techniques for modification of calcified coronary lesions to gain the best PCI results.









