Introduction
Head and neck squamous cell carcinoma (HNSCC) accounts for 0.2 million cases every year in India. Of these, oropharyngeal squamous cell carcinoma (OPSCC) comprise 20,000 cases per year [1]. Concurrent chemoradiotherapy (CCRT) is the current standard of care for locally advanced OPSCC. Treatment intensification with CCRT has led to increased acute and late toxicities [2–4]. There are several predictive factors for development of radiation morbidities, such as biologically effective dose, radiation techniques, and use of concurrent chemotherapy [5]. However, these factors are not sufficient to explain the inter-individual variability among patients for development of radiation toxicities. Gene modifications in biological pathways like DNA repair, cell cycle, and apoptosis have been found to be predictive of acute radiation toxicities in HNSCC like XRCC, RAD51, GSTP, etc. [6, 7]. Furthermore, the immune system of the patient is instrumental in determining radiation repair, and immune escape mechanisms may determine the individual response to radiation therapy (RT). Identification of novel biomarkers modulating immune response, like cytotoxic T lymphocyte-associated antigen 4, lymphocyte-activation gene-3, T-cells immunoglobulin mucin protein-3, and programmed death receptor ligand 1 (PD-L1), may predict the radiation response and toxicities in HNSCC [8].
Programmed death receptor 1 (PD1) is a member of the extended family of T-cell regulators expressed on the surface of activated T-cells, B-cells, and macrophages, and its ligand PD-L1 is a cell surface glycoprotein expressed on T-cells, macrophages, cancer cells, and other tissues. Co-expression of PD-1 and PD-L1 inhibits proliferation of lymphocytes and T-cells mediated cytokine secretion. The PD-1/PD-L1 interaction protects healthy cells from excessive inflammatory response. However, in the tumour microenvironment (TME) this interaction causes inhibition of activated T-cell proliferation and promotes apoptosis of T-cells, resulting in enhanced tumour cell growth [9].
The critical role of PD-L1 in HNSCC carcinogenesis was demonstrated by Zheng et al. [10]. Recent studies suggest that the PD-1/PD-L1 axis is closely related to HPV-associated HNSCC, suggesting the relevance of PD-L1 expression on tumour cells (TCs) and T-cells in OPSCC [11, 12]. The association of PD-L1 expression with clinicopathological features and its prognostic significance remains controversial [13–16] . While some studies have reported poor clinical outcomes [14, 17] in PD-L1 expressing HNSCC, others have found improved survival [8, 15]. Although some studies demonstrate a change in PD-L1 expression level by local fractionated RT [18, 19], the correlation of PD-L1 expression with radiation toxicities remains unknown. So, we conducted this prospective study to evaluate the correlation of PD-L1 expression in tumour and tumour infiltrating lymphocytes (TILs) with acute radiation toxicity and response in patients with OPSCC treated with definitive CCRT.
Material and methods
This was a single-institution prospective observational study conducted from Dec 2017 to March 2021. The enrolment period was from Jan 2018 to April 2019. The study protocol was approved by the Institutional Ethical Committee (IEC no. 29/17). It included patients with histopathologically proven squamous cell carcinoma of the oropharynx aged between 18 and 80 years, with stage II to IVA disease, Karnofsky performance status ≥ 70, and normal haematological, renal, and hepatic functions. Patients were required to sign an informed consent form before enrolment in the study. Pre-treatment workup included baseline haematological parameters, chest X-Ray PA view, contrast-enhanced computed tomography (CECT) of face and neck region, and direct laryngoscopy. P16 expression was tested and patients were staged according to American Joint Committee on Cancer recommendations (eighth edition 2018) [20].
Immuno-histochemistry for PD-L1 expression
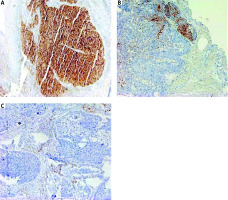
Biopsy samples or slide/blocks were sent to the pathology department of the institute. The tissue samples were formalin fixed and paraffin embedded. Then 3–4 µm sections were cut and subsequently stained with haematoxylin and eosin to check for the presence of tumour. Immuno-histochemistry (IHC) was applied using antibodies to PD-L1 (clone SP263) in the automated IHC slide staining system, VENTANA BenchMark XT according to manufacturer’s instructions. Deparaffinization, retrieval, and staining were done onboard (Ventana Medical Systems, Inc., Tucson, USA). Programmed death receptor ligand 1 expression was recorded on TCs and TILs. Programmed death receptor ligand 1 expression was considered positive if >1% of cells showed moderate to intense membranous or cytoplasmic staining with grading as follows: 1–10% (1+); 11–50% (2+), and > 50% (3+). The absence of membranous expression of TCs was deemed to be PD-L1 negative. Programmed death receptor ligand 1 expression in TILs (PD-L1-TILs) was reported separately (Fig. 1).
Fig. 1
Immunohistochemical staining for programmed death receptor ligand 1 (PD-L1) in patients with squamous cell carcinoma oropharynx. PD-L1 expression scored 3+ (strong membrane staining in more than 50% of tumour cells [TCs]) (A), PD-L1 expression in tumour cell scored 1+ (weak to moderate expression in 10% TCs) (B), PD-L1 expression in 2% TCs and 10% in tumour infiltrating lymphocytes (C)
Treatment
Definitive CCRT with curative intent was given to all patients depending on their clinical stage and general condition. Contrast-enhanced computed tomography simulation was performed using appropriate thermoplastic cast immobilization, and images of 3 mm slice thickness were acquired. Data were transferred to a treatment planning system (XIO Version 5.0/MONACO version 5.11.02) using DICOM (Digital Imaging and Communications in Medicine) protocol 3.0. Delineation of target volumes and organ at risk volumes were performed as defined in International Commission on Radiation Units and Measurements report numbers 50 and 62 [21]. A target dose of 66 to 70 Gray (Gy) in 33 to 35 fractions in 2 phases over a period of 6 to 7 weeks, 5 days a week was planned using a shrinking field technique.
In the first phase, 44 Gy was delivered using a parallel opposed field to bilateral face and neck and a low anterior neck field. The second phase was delivered using an off-cord bilateral parallel opposed field with or without posterior neck electron boost to a dose of 22–26 Gy. Posterior neck was boosted with 6–12 MeV electrons using R-90 as prescription isodose line if clinically indicated. Set up verification was done with EPID (Electronic Portal Imaging Device) twice a week to ensure proper positioning during the entire course of RT. Treatment delivery was done using a linear accelerator (Infinity, Synergy and Compact; Elekta) having MLC with leaf width of 1 cm using 6 MV photons. Injection of cisplatin 40 mg/m2 weekly was given as concurrent chemotherapy. Patients were assessed at least once during RT, and toxicities were recorded by the physician weekly as per Radiation Therapy Oncology Group (RTOG) acute toxicity criteria RTOG [22].
Post-treatment visits were monthly for 3 months and then 2-monthly for the next 6 months and every 3 months thereafter. Three months after completion of treatment, or earlier if clinically indicated, response assessment was done using clinical examination, direct laryngoscopy, and CECT of the face and neck with or without biopsy or fine needle aspiration cytology of suspicious or persistent lesions. In accordance with World Health Organization (WHO) response assessment criteria, the response was classified into complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) [23]. A schema of the study is presented in Figure 2.
Statistical analysis
Statistical analysis was done with statistical package for social sciences (SPSS, version 23.0, IBM). Mean and standard deviation were estimates of quantitative data. Programmed death receptor ligand 1 expression was correlated with the clinic-pathological parameters and response to treatment using Student’s t-test or χ2 test. WHO response was correlated with PD-L1 expression using χ2 test or Fisher exact test. Overall survival (OS) was calculated from date of registration until the date of death from any cause. Progression-free survival (PFS) was evaluated from the date of initiation of treatment until the date of disease progression (locoregional, distant, or both) or death. Survival rates were estimated using Kaplan-Meier method, and the log-rank test was used to compare survival outcomes. All reported p-values were 2-sided, and p ≤ 0.05 was considered significant.
Results
In total, 56 patients were enrolled, of whom 51 successfully completed treatment and were included in the study cohort. Three patients defaulted before initiation of treatment (due to personal reasons), and 2 patients expired during treatment (due to pneumonia and cardiac arrest respectively), and these 5 patients were excluded from the study.
Ten (19.6%) patients were positive for p16 expression. Twenty (39.2%) patients had PD-L1 expression in TCs (PD-L1-tumour +ve). Eight (15.7%) patients had 1+ PD-L1 expression, while 6 (11.8%) patients had 2+ and 3+ expression each. The median age was 55 years (range, 26–75 years). Clinicopathological correlation of PD-L1 expression on TCs with patient characteristics is summarized in Table 1. Eighteen (35.3%) patients had PD-L1 positivity in TILs (PD-L1-TILs +ve) and 11 (21.6%) had PD-L1 expression in both TCs and TILs. PD-L1 expression in TCs was significantly associated with PD-L1 expression in TILs (p = 0.034). Higher nodal burden was observed in PD-L1-TIL –ve than PD-L1-TILs +ve (65% vs. 44%, p = 0.03).
Table 1
Comparison of baseline patient characteristics stratified by the programmed death receptor ligand 1 expression in tumour cells
The median RT dose was 70 Gy (range, 66–70 Gy), with a median RT duration of 50 days (range, 48–57 days). All patients received cisplatin-based CCRT with a median cumulative dose of 320 mg (range, 250–350). Forty-four (86.3%) patients received ≥ 5 cycles of CCRT. Seven patients received ≤ 4 cycles; of these, 2 were PD-L1-tumour +ve. Two patients received 68 and 66 Gy over 34 and 33 fractions, respectively, due to grade 4 mucositis; both were PD-L1-tumour –ve. Prophylactic nasogastric tube (NGT) or percutaneous endoscopic gastrostomy tube feeding was not used as per institutional policy. Nasogastric tube feeding was required in 6 patients, 4 in PD-L1 –ve group and 2 in PD-L1 +ve group (1 each in tumor and TILs). The median duration of NGT feeding was 25 days (18 days for PD-L1 +ve and 22 days for PD-L1 –ve). Highest grade of radiation toxicity observed during CTRT were taken into consideration. Radiation toxicities in PD-L1 +ve vs. PD-L1 –ve patients are compared in Table 2. On multivariable modelling, PD-L1 status was not found to be an independent predictor for severe acute radiation toxicities (all p-value > 0.05).
Table 2
Correlation of the programmed death receptor ligReferences and 1 expression in tumour and tumour-infiltrating lymphocytes with acute radiation toxicity
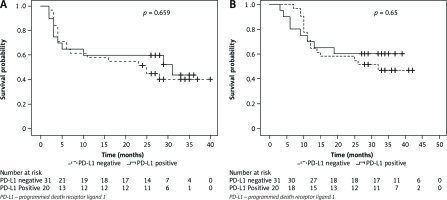
On response evaluation after 3 months of RT completion, overall CR was achieved in 67% patients while 24% had PR and 4% had PD, none of the patients had SD. Complete response was 76% vs. 67% and PR + PD was 23% vs. 10% respectively in PD-L1-tumour +ve vs. PD-L1-tumour –ve group (p = 0.74). At a median follow-up of 25 months (range,1–39 months), the 2-year OS rate of entire cohort was 58.8% and the 2-year PFS rate for entire cohort was 54.9% (median PFS – 28 months). Two-year OS rate for PD-L1-tumour –ve vs. PD-L1-tumour +ve seen in other tumour types and group was 58.1% and 60% [hazard ratio (HR), 1.21; 95% CI: 0.51–2.83; p = 0.65]. Two-year PFS rate was 51.6% in PD-L1-tumour –ve group v 60% for PD-L1-tumour +ve group (HR, 1.19; 95% CI: 0.54–2.58; p = 0.659) (Fig. 3).
Fig. 3
Kaplan-Meier’s analysis of survival rates. Progression-free survival (A), overall survival of tumour programmed death receptor ligand 1 positive vs. survival probability (B)
Complete response rate was 59% vs. 77% and PR + PD was 41.4% vs. 22.4%, respectively, in the PD-L1-TILs +ve vs. the PD-L1-TILs –ve group (p = 0.20). The two-year OS rate for the PD-L1-TILs –ve group vs. the PD-L1-TILs +ve group was 60.6% and 55.6%, respectively (HR, 0.829; 95% CI: 0.36–1.89; p = 0.65). The two-year PFS rate was 54.5% in the PD-L1-TILs –ve group vs. 55.6% in the PD-L1-TILs +ve group (HR, 1.21; 95% CI: 0.568–2.59; p = 0.619).
Discussion
The PD-1/PD-L1 axis has emerged as a valuable biomarker in predicting response to anti PD-1/PD-L1 therapy. The PD-L1 positivity in HNSCC ranges from 18–80% reported in various studies (11–15, 22). This high variability is also seen in other tumour types and is due to technical difficulties in determining PD-L1 expression [24, 25]. In our study, positive PD-L1 expression in TCs and TILs was observed in 39.2% and 35.2%, respectively, which is in concordance with contemporary studies. Although HPV status has been shown to have positive correlation with PD-L1 expression [26], Kim et al. [16] could not find any association between them. In our study no significant association of PD-L1 expression with clinicopathological parameters and HPV status was observed. However, in our study PD-L1 expression in TILs was significantly associated with N stage and PD-L1 expression in tumour.
Our study, to the best of our knowledge, is the first prospective study to correlate PD-L1 expression with both acute radiation toxicity and treatment response in patients of OPSCC treated with cisplatin based CCRT. We noted higher grade ≥ 3 maximal toxicities in terms of oral mucositis, dysphagia, xerostomia, and laryngeal toxicity in the PD-L1-tumour –ve group. Similar findings were also observed in PD-L1-TILs because grade ≥ 3 oral mucositis; dysphagia, and grade ≥ 2 skin reactions were more common in the PD-L1-TILs –ve group (Table 2). The programmed death receptor ligand 1 negative patients also had delayed healing of radiation reactions in comparison to their positive counterparts one month after completion of RT.
The immune effect of fractionated RT elucidates our finding. Radiation causes generation of reactive oxygen species and nitric oxide in the TME and breaks the DNA double strand, leading to release of cytokines, activation of toll-like receptors signalling immune response pathway, and secretion of pro-inflammatory cytokines such as interleukin-1 (IL-1), IL-2, -4, and -6, and tumour necrosis factor, leading to activation of immune cells. These mediators increase vascular permeability causing more infiltration and recruitment of inflammatory cells. This leads to proliferation of regulatory and helper T-cells. The dead cells at the site of irradiation also release damage-associated molecular patterns like adenosine triphosphate, which activate local dendritic cells, increasing antigen presentation [27–29]. As demonstrated by Dovedi et al. [18], the PD1/PD-L1 expression in immune and TCs is upregulated by RT, which leads to functional anergy of T-cells and hence modulation of acute inflammatory response. This could be a plausible hypothesis suggesting fewer acute radiation morbidities in patients with increased PD-L1 expression. These findings concur with our finding of decreased radiation toxicity in patients with PD-L1 expression. Myers et al. also reported enhanced healthy tissue damage by T-cell activation with the addition of PD-1 blockade to RT in mice treated with RT and PD-1 blockade vs. RT alone [30].
The correlation of CCRT and PD-L1 expression has not been extensively studied. In a retrospective study of 92 patients by Fukushima et al. [31] better outcomes were observed in patients with high PD-L1 expression. A caveat to their study was the inclusion of patients who received induction chemotherapy and various CCRT regimens, unlike our study which has a homogenous treatment cohort. We observed better CR in PD-L1-tumour +ve patients, but this was statistically not significant (76% vs. 67%, p = 0.74). The impact of PD-L1 expression on clinical outcomes of patients has been studied in various solid tumours, but the correlation with prognosis remains to be deciphered. Two Meta-analysis done in HNSCC by Li et al. and Yang et al. could not establish a correlation of PD-L1 expression with OS and DFS; however, in the former, PD-L1 +ve patients showed improved PFS, while poor survival in the Asian population was observed in the latter [32, 33]. Our results could not show significant association of PD-L1 expression on tumour or TILs with OS and PFS in OPSCC patients. Larger cohort-based studies with longer follow-up are needed to establish an association.
The programmed death receptor ligand 1 mediates the inter-relationship between TCs and TILs. However, the PD-L1 upregulation could be an adaptive response resulting from a vigorous immune microenvironment which evinces anti-cancer effects [34–36]. Multiple cell types like malignant, immune, and stromal cells and immune checkpoints impose more intricacy to TME (9,31,32). Most studies show that the pathogenesis of OPSCC (especially HPV positive) has an immunosuppressive origin and is related to the PD-1/PD-L1 pathway, but it is not yet established whether this pathway plays a greater role in HPV-positive cancers compared to HPV-negative ones [11, 16]. Therefore, PD-L1 expression alone may not be considered a strong prognostic factor in OPSCC.
A major limitation to our study was the small sample size and a shorter follow-up. Due to lack of specific data on the study outcomes and owing to limited resources, a sample size was also not specifically calculated for our study and hence the exact power of our study is unknown, and this is a limitation of our study. Owing to the small sample size, only qualitative expression of PD-L1 could be taken into consideration, so the difference in toxicity and outcome due to intensity of PD-L1 expression could not be established. The number of HPV positives was also very low, perhaps because of the regional population of patients included in our study.
Despite these shortcomings, we believe that the scarcity of data regarding PD-L1 expression in OPSCC and its effect on radiation toxicities in patients makes our study unique. The majority of the studies [18, 29] focused on effect of RT on changes in PD-L1 expression and the role of PD-L1 blockade therapy [37] in the outcome, but none of these discussed the outcome of RT in terms of toxicity and treatment response in patients with positive PD-L1 expression. Prospective use of PD-L1 expression as a predictor of radiation toxicities may guide early institution of supportive therapy and early consideration of NGT insertion or percutaneous endoscopic gastrostomy tube placement in patients having negative PD-L1 expression. This could reduce toxicity-related treatment interruption and decrease morbidity in patients, which will further lead to better treatment outcomes. This could serve as a potential biomarker for predicting acute radiation toxicities in this cohort of patients. The combination of immune check-point inhibitors with radiotherapy in HNSCC is an intensive area of ongoing research [38], and the findings from our study may guide future clinical trials in this setting.
Conclusions
Patients with positive PD-L1 expression in TCs and TILs treated with definitive CCRT have significantly fewer acute radiation toxicities compared to their negative counterparts. The programmed death receptor ligand 1 expression alone could not be considered as a prognostic factor for survival in OPSCC. Our study provides encouraging data for the use of PD-L1 expression as a potential biomarker for acute radiation toxicities, but it requires further evaluation in a larger cohort to find its prognostic implication on clinical outcome in terms of tumour response and survival. This may help in the recognition of the cohort of patients who may need early and additional support during treatment.









