Introduction
Pathogens, damaged cells, irritation, stress, some medications, or immunizations can all cause inflammation, which is a typical biological response of the immune system (Chen et al., 2017). Inflammation is involved in the pathological events of nearly all human infections, diseases, and cancers. Unresolved inflammation has been associated with a number of life-threatening diseases, such as diabetes, cardiovascular disease, rheumatoid arthritis, acute respiratory distress syndrome, Alzheimer’s disease, autoimmune system disorders including inflammatory bowel disease and rheumatoid arthritis, among others (Chen et al., 2017; Ginwala et al., 2019). Increased morbidity and mortality in a number of microbial and viral infections have been primarily attributed to uncontrolled inflammation (Adeboboye et al., 2020).
When administered to the host in sufficient proportions, some lactic acid bacteria (LAB) can have a number of positive health effects (Azad et al., 2018). They are primarily nonpathogenic and have been consumed with food over time. According to some studies, some LAB may have anti-inflammatory and immunoregulatory characteristics (Amdekar et al., 2012). Many species of LAB, such as Lactobacillus casei, Lactobacillus fermentum, Lactobacillus delbrueckii, Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus reuteri, possess potential therapeutic properties as they can prevent the development of some diseases, as shown mostly using animal models (de Moreno et al., 2011; Zhao et al., 2019; Minj et al., 2021). However, the involvement and potential mechanisms of important anti-inflammatory cytokines in the resolution of inflammatory reactions remain unexplored. The immune system’s homeostasis is controlled by inhibitory cytokines such as interleukin-10 (IL-10) and transforming growth factor-β (TGF-β). They have been described as being generated by immune cells stimulated by LAB and are essential to the regulation of the inflammatory response of the body (Komai et al., 2018) (de Moreno et al., 2011).
The World Health Organization (WHO) has estimated that between 70 and 80% of the world’s population receive their primary medical care from nonconventional medicine primarily derived from herbal sources (WHO, 2002; Roy et al., 2011). In contrast, some people receive nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin, ibuprofen, ketoprofen, diclofenac, indomethacin, etc., which inhibit COX-2 enzymes. However, these medications have a wide range of adverse effects, of which the most severe is gastrointestinal toxicity. Other side effects include bleeding tendencies, ulcer aggravation, throat swelling, and liver or kidney issues (Shobana et al., 2017).
Therefore, this study was designed to evaluate the anti-inflammatory-associated immune regulatory activities of LAB isolated from two locally fermented food products in Nigeria using a carrageenan-induced acute inflammatory model in Wistar rats, with a specific focus on anti-inflammatory cytokine production profile to search for alternatives to conventional NSAIDs that have deleterious effects in some individuals.
Materials and methods
Collection of samples
Guinea corn grains were purchased from the Oja-Oba market (longitude 5.2058 and latitude 7.2571) in Akure, Ondo State, Southwest Nigeria, and prepared as fermented slurry under aseptic conditions as described by Adebolu et al (2007). The indigenous dairy product “Nunu” from Nigeria was also purchased from a local farm in the Gaga community (longitude 5.1901 and latitude 7.2459) in Akure, Ondo State, Southwest Nigeria. The samples were stored in sterile containers and immediately transported to the laboratory for bacteriological analysis under refrigerated conditions in dry ice chests.
Isolation and identification of bacteria
LAB were isolated from fermented guinea corn slurry and “Nunu”. LAB were enumerated and isolated on MRS agar using the pour plate method and incubated anaerobically at 37°C for 48 h using standard microbiological methods, as described by Bin-Masalam et al. (2018). Preliminary identification of isolates was conducted by morphological and conventional biochemical characterization. Molecular identification of the isolated LAB was carried out by harvesting cell pellets from 2 ml samples of overnight cultures (up to 2 × 109 bacterial cells) of each LAB isolate grown in MRS broth, and then, DNA was extracted using the Bacteria DNA Preparation Kit (Jena Bioscience GmbH, Jena, Germany) according to the manufacturer’s instructions. Nearly the entire region of the 16S rDNA gene was amplified using polymerase chain reaction (PCR). Using the primer pairs 27F-YM (5′–AGA GTTTGATCCTGGCTCAG–3′) and 1492r (5′–TACCTTGTTACGACTT–3′), the 16S rDNA gene of the bacteria was amplified (Frank et al., 2008). On a 1.5% agarose gel, the amplification product was separated, and electrophoresis was carried out at 80 V for 1 h 30 min. After electrophoresis, DNA bands were visualized by ethidium bromide staining. As a DNA molecular weight marker, 100-bp DNA Ladder Ready to Load (Solis, BioDyne Tartu, Estonia) was used. The ExoSAP-ITTM PCR product cleanup reagent (Thermo Fisher Scientific, USA) was used to purify PCR products prior to DNA Sanger sequencing, and the ABI Sequencing Analysis software was used to analyze the data (version 5.2). The obtained sequences were BLAST-searched to detect similar sequences in the NCBI database (https://www.ncbi.nlm.nih.gov).
Drugs and chemicals used
Iota-carrageenan (CAS 9064-57-7) was bought from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), diclofenac sodium was purchased drug from Impulse Pharma Pvt. Ltd. (Boisar, India), and cytokine ELISA kits: Rat CReactive Protein (CR-P), CRP ELISA Kit (CAT NO: EKRAT-0459), Rat IL-10, IL-10 ELISA Kit (CAT NO: EKRAT-0650), and Rat TGF-β (CAT NO: EKRAT-0789), were purchased from MELSIN Medical Co. Ltd. (Changchun, China).
Experimental animals and treatment groups
Healthy male Wistar rats (8–10 weeks old) with an average weight of 150 g used in this study were acquired from the Department of Animal Production and Health, FUTA, Nigeria. The rats were fed with water and standard commercial feed diet purchased from a certified veterinary clinic in Akure, Nigeria (feed composition: hydrolyzed maize starch (65%), fish meal (22%), palm oil (5%), bone meal (2.5%), salt (0.5%), and cellulose (5%)), and acclimatized for 1 week before the experimental session. All experimental procedures were carried out following the guidelines of the University’s Research Ethical Committee (FUTA/ETH/21/06). The animals were divided into seven groups of three rats each: group A, general control, was not injected with carrageenan and not given any treatment; group B received carrageenan only without any treatment (negative control); groups C, D, E, and F were administered carrageen and were orally treated with 5 × 107 CFU/ml of L. fermentum CIP 102980, L. fermentum NBRC 15885, L. plantarum CIP 103151, and Lactobacillus pentosus 124-2, respectively, after the development of paw edema in the rats. Group G was administered carrageenan and treated with diclofenac sodium (150 mg/kg body weight). This group served as the positive control.
Carrageenan-induced acute inflammation in rat paw tissues
Except for the general control group, acute inflammation was induced by subplantar injection of 1 ml of 1% Iota-carrageenan dissolved in sterile saline into the right hind paws of all rat groups. The thickness of rat paws was measured at 20 min before carrageenan injection and then at different time intervals (0, 1, 4, 8, 24, 72, 168, and 336 h) using a digital Vernier caliper and was measured in millimeters (mm) (Amdekar et al., 2012).
Blood sample collection
Blood samples (about 1.5 ml) were collected from rats bled at the aforementioned intervals through cardiopuncture following the guidelines of the Institutional Animals Ethics Committee, the Federal University of Technology Akure, Nigeria, Centre for research and development. Ethical permission was obtained from FUTA Research Ethical Committee (FUTA/ETH/21/06). Differential white blood cell (WBC) and cytokine ELISA analyses were carried out on the collected blood samples. The blood samples were allowed to coagulate for 60 min at room temperature and then centrifuged at 1500 g for 15 min, and the crude serum was stored in new cryovial tubes. The harvested serum was stored at –20°C until further use.
Differential WBC count
To calculate the differential WBC count, a drop of blood sample was placed on a clean glass slide, and then, a thin film was prepared and allowed to dry. The thin film was stained with Leishman stain for 10 min, fixed, dried, and then viewed under a microscope. Leucocytes were counted in a field based on their morphology.
Myeloperoxidase (MPO) activity
The MPO activity was then assessed using the procedure of Eddouks et al (2012). Aliquots (5 ml) of supernatants of the right hind paw tissue homogenate were collected from each rat and were mixed with PBS (pH 7.4, 15 ml), NaH2PO4 (0.22 M, pH 5.4, 2 ml), H2O2 (0.026% (v/v), 2 ml), and tetramethyl benzidine (18 mM in 8% (v/v) aqueous dimethyl formamide, 2 ml). The enzyme activity of each reaction mixture was assessed at 620 nm following its reaction with sodium acetate (1.46 M, pH 3.0, 3 ml) for 10 min at 37°C.
Cytokine assay
CRP levels (a proinflammatory biomarker) (ng/ml), IL-10, and TGF-β (anti-inflammatory cytokines) (pg/ml) were calculated using an ELISA analyzer reader (Labtech Auto ELISA P, Mumbai, India). Serum samples were used to carry out the assay following the method of Valentini et al. (2015).
Statistical analysis
Data were expressed as mean ± standard error mean calculated over independent time frames of experiments carried out in triplicate. One-way analysis of variance was used, followed by a post hoc test, and Bonferroni’s multiple-comparison test for the difference between treatment groups compared with control (group B) using GraphPad Prism, version 5.0.
Results
Isolation and identification of Lactobacillus
Based on the morphological and biochemical characterizations examined in this study, four Lactobacillus species were initially identified. The two L. fermentum strains were from the “Nunu” samples, whereas L. plantarum and L. pentosus were from the fermented guinea corn slurry. The four LAB were identified as L. fermentum CIP 102980, L. fermentum, L. plantarum CIP 103151, and L. pentosus 124-2 based on molecular characterization, as shown in Table 1a and b.
Table 1A
Colonial morphology and biochemical characteristics of lactic acid bacteria isolated from “Nunu” and fermented guinea corn slurry
Table 1B
Molecular identities of lactic acid bacteria isolated from “Nunu” and fermented guinea corn slurry
Carrageenan-induced inflammation in rat paw tissues
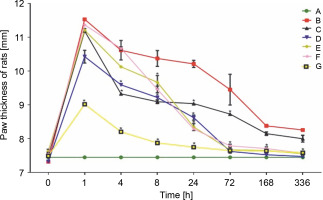
Carrageenan was injected into the right hind paw, causing progressive edema that peaked in the first hour after injection. Paw thickness (7.45 ± 0.00 mm initial) in group A rats remained unchanged throughout the experiment. The highest increase in paw thickness (11.54 ± 0.25 mm) was observed in untreated group B rats after the first hour of injection. A significant decrease was only observed after 24 h, till day 14, in this rat group. However, the paw volume of LAB-treated rats in the remaining groups showed a rapid gradual decrease after 4 h of treatment. Rats treated with L. fermentum NBRC 15885 (group D) showed a consistent decrease in paw size (7.48 ± 0.10 mm at 336 h) compared with other treatment groups. The diclofenac-treated group showed a lesser increase (9.02 ± 0.02 mm) in paw volume after the first hour compared with all other groups as shown in Figure 1. Data are expressed as mean ± standard error of three rats per group (n = 3). Values were statistically significant at P < 0.05* indicates a significant difference as compared to group B (negative control). Group A: general control; Group B: carrageenan only (negative control); Group C: fed with Lactobacillus fermentum CIP 102980; Group D: fed with Lactobacillus fermentum NBRC 15885; Group E: fed with Lactobacillus plantarum CIP 103151; Group F: fed with Lactobacillus pentosus 124-2; Group G: treatment with diclofenac sodium (positive control). Values were statistically significant at P < 0.05.
Total circulating levels of immune cells in LAB-treated and untreated rats
Rats that did not receive a carrageenan injection or the treatment maintained a steady level of circulating immune cells throughout the experiment. All LAB-treated groups showed a significant decrease in the proportion of circulating lymphocytes 1 h post-treatment, with group F showing the highest reduction (60.50 ± 2.89%). Circulating lymphocytes in inflamed, untreated group B rats decreased steadily at 1, 8, and 24 h, increasing slowly at 72 h. As shown in Table 2A the recovery of circulating lymphocytes in the LAB-treated groups started gradually 24 h after treatment, and an almost complete recovery was achieved by 72 h, with a range of 73.67 ± 3.48–80.00 ± 0.00%. Rats from group B showed the highest infiltration of neutrophils in the blood circulation, at 51.00 ± 1.72%, after 24 h (peak time). The number of infiltrating neutrophils in the rats subjected to LAB treatment was reduced in their blood circulation, with group D rats having the lowest concentration of neutrophils (27.67 ± 1.45%) at the expected neutrophil response peak time of 24 h (P < 0.05). In all tested rat groups, only a slight increase in the monocyte count was observed. Group B rats also showed a slight increase in the number of circulating monocytes of 2.00 ± 0.00 and 2.33 ± 0.33% at 4 and 24 h, respectively, although it was not significant at P < 0.05. Group G rats showed a high monocyte count of 3.00 ± 0.58% 4 h postinjection, which was similar in effect to group C and D rats, as shown in Table 2C.
Table 2A
Effect of oral administration of lactic acid bacteria on total circulating lymphocytes [%]
Table 2B
Effect of oral administration of lactic acid bacteria on total circulating neutrophils [%]
Table 2C
Effect of oral administration of lactic acid bacteria on total circulating monocytes [%]
[i] Data are expressed as mean ± standard error of three rats per group; group A – carrageenan control; group B – carrageenan only (negative control); group C – fed with Lactobacillus fermentum CIP 102980; group D – fed with Lactobacillus fermentum NBRC 15885; group E – fed with Lactobacillus plantarum CIP 103151; group F – fed with Lactobacillus pentosus 124-2; group G – treatment
MPO activity
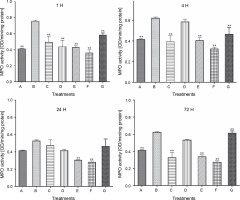
Figure 2 demonstrates that during the first hour of injection and therapy, the MPO activity of the carrageenan rat control group (B) significantly increased to 0.76 ± 0.0120 (P < 0.05). However, oral administration of LAB species significantly reduced its activity in all LAB-treated rat groups for 72 h. This was observed as an early response in all LAB-treated groups; however, group E and F rats showed a more consistent and continuous decline in MPO activity for 72 h, with the only deviation from this pattern observed in the L. fermentum CIP 102980-treated group D rats at 4 and 72 h.
Fig. 2
Effect of lactic acid bacteria treatments on carrageenan-induced activity of myeloperoxidase in rat paw edema at intervals; A – carrageenan control, B – carrageenan only (negative control), C – Lactobacillus fermentum NBRC 15885, D – Lactobacillus fermentum CIP 102980, E – Lactobacillus plantarum CIP 103151, F – Lactobacillus pentosus 124-2, G – diclofenac sodium (positive control); data represented the mean ± S.E.M. (n = 3); ** P < 0.005 compared with the carrageenan control group
Proinflammatory biomarker (CR-P)
Rats from all groups tested produced CR-P (ng/ml), which peaked at 8 h. However, the level of CR-P in rats from control group A remained at 300.00 ± 0.00 ng/ml throughout the experiment. However, all rat groups, except for those treated with L. fermentum NBRC 15885 (group D), showed a substantial increase in rat serum CR-P levels at 8 h. At 8 hours, group F rats showed the highest CR-P levels (6064.00 ± 286.75 ng/ml), whereas group D rats showed the least (2411 ± 22.52 ng/ml). CR-P levels of all groups were significantly lower than the average range for rats of 2000 ng/ml at 24, 72, and 168 h (Fig. 3).
Fig. 3
Production of proinflammatory biomarker C-reactive protein (ng/ml) in rats treated with lactic acid bacteria
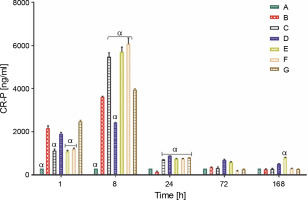
Data are expressed as mean ± standard error of three rats per group (n = 3). Values were statistically significant at P < 0.05. α indicates a significant difference as compared to group B (negative control). Group A: carrageenan control; Group B: carrageenan only (negative control); Group C: fed with Lactobacillus fermentum CIP 102980; Group D: fed with Lactobacillus fermentum NBRC 15885; Group E: fed with Lactobacillus plantarum CIP 103151; Group F: fed with Lactobacillus pentosus 124-2; Group G: treatment with diclofenac sodium (positive control).
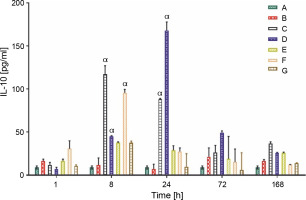
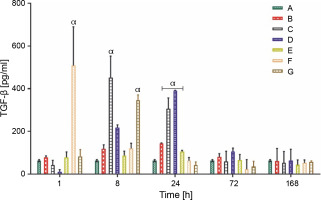
Production of anti-inflammatory cytokine (IL-10 and TGF-β)
The IL-10 and TGF-β levels in group A rats that did not receive carrageenan injection or treatment remained constant throughout the experiment, at 9.00 ± 1.00 and 62.90 ± 3.00 pg/ml, respectively. All rat groups, except for groups A and B, showed an initial increase in the production of IL-10 (pg/ml). Group C rats showed a significantly higher production of IL-10 (117.00 ± 10.03 pg/ml) at 8 h, whereas the overall peak was observed in group D rats (167.80 ± 10.01 pg/ml) at 24 h after LAB treatments. Groups C and D showed a sustained average serum IL-10 level between the ranges of 35.00 and 50.00 pg/ml at 72 and 168 h after feeding with lactic acid bacteria (Fig. 4A). The production of TGF-β, a complimentary anti-inflammatory cytokine, was highest in group F rats (508.40 ± 182.00 pg/ml) at the first hour, group C (452.60 ± 104.03 pg/ml) at 8 h, and group D (390.60 ± 2.01 pg/ml) 24 h post-treatment, as shown in (Fig. 4B).
Data are expressed as mean ± standard error of three rats per group (n = 3). Values were statistically significant at P < 0.05. α indicates a significant difference as compared to group B (negative control). Group A: carrageenan control; group B: carrageenan only (negative control); group C: fed with Lactobacillus fermentum CIP 102980; Group D: fed with Lactobacillus fermentum NBRC 15885; Group E: fed with Lactobacillus plantarum CIP 103151; Group F: fed with Lactobacillus pentosus 124-2; Group G: treatment with diclofenac sodium (positive control).
Discussion
Carrageenan is a strong phlogistic chemical used in the release of inflammatory and proinflammatory mediators such as prostaglandins, histamine, bradykinin, and some specific cytokines (Castardo et al., 2008; Amdekar et al., 2012). Carrageenan-induced rat paw edema model has been explored as a classic method in medicine for investigating drugs with anti-inflammatory potential (Silver et al., 2010).
Acute inflammation develops in two stages. When a substance like carrageenan was injected, the first phase, which results in fluid accumulation in endothelium tissues, was triggered by the release of inflammatory mediators such as histamine, serotonin, and kinins. The second phase began a few hours later with the further release of prostaglandins and associated substances. This was also characterized by leukocyte infiltration (especially primary response from neutrophils and monocytes) at the infection or damaged site following the activation of pattern recognition receptors like Toll-like receptors, which are activated by cytokine production in a series of inflammatory cascades (Silver et al., 2010; Amdekar et al., 2012).
Different species of Lactobacillus were identified based on the results of the morphological and biochemical characterization of the primary LAB isolated from the two samples (Nunu and Ogi) analyzed in this study. Molecular analysis led to the identification of four strains: L. plantarum CIP 103151, L. fermentum CIP 102980, L. fermentum NBRC 15885, and L. pentosus 124-2. The presence of L. fermentum in milk has been reported in several studies, and it was shown to be most abundant in a total of 373 LAB isolated from spontaneously fermented “Nunu” in Ghana (Akabanda et al., 2013). In the past 40 years, several cytokines, including IL-6, TNF-α, IL-10, and TGF-β, have been detected in milk (Brenmoehl, 2018). They have been associated with the presence and interactions of the probiotic LAB strains, and their importance in the maturation of the immune system from developing gastrointestinal tracts has been reported (Ménard et al., 2008; Brenmoehl, 2018). L. plantarum has also been reported to dominate the microflora of Ogi and is considered safe, with some reports indicating the presence of closely related L. pentosus species (Adisa and Enujiugha, 2020). The dominance of L. plantarum has been related to its ability to withstand acids (pH 4.6–4.8) (Okoronkwo, 2014). The results of the LAB treatment of the inflammatory model showed that L. fermentum CIP 102980, L. plantarum CIP 103151, L. pentosus 124-2, and L. fermentum NBRC 15885 significantly reduced the influx of neutrophils and monocytes in LAB-treated rats, which is necessary for the sustained release of inflammatory mediators. This may have led to the significant decrease observed in paw thickness in groups C, D, E, and F. However, throughout the experimental stages, L. fermentum NBRC 15885 showed the most significant and consistent anti-inflammatory properties, whereas L. pentosus 124-2 demonstrated the least. Previous studies have reported the anti-inflammatory activity of Lactobacillus in the gut of different animals (Amdekar et al., 2012; Azad et al., 2018).
Neutrophils play a significant role in sustaining the inflammatory process as the accumulation of neutrophils aids the release of more proinflammatory mediators and cytokine and oxidative burst leading to stress at the inflammation site. MPO, which serves as a common in vivo index of inflammation, neutrophil infiltration (in this case to the rat paw), and an important marker of oxidative stress (Eddouks et al., 2012; Li et al., 2013), revealed that LAB species remarkably suppressed MPO activity in inflamed rat tissues. This provides an important confirmatory rationale for the mobilization of neutrophils earlier observed in serum. The impact of the LAB treatment on MPO activity also had an early response, which is consistent with other findings of the present study and similar to the findings of Li et al. (2013). The decrease in the generation of proinflammatory cytokines could have been explained by the decreased neutrophil activity and concomitant increased MPO activity (Haegens et al., 2009). The upregulation of anti-inflammatory cytokines during the alleviation of inflammation may also have a direct impact.
Naturally, the immune system of the body controls inflammation through complex immune signaling that involves CD4+ T regulatory cells and uses many suppressor mechanisms, such as the production of IL-10 and TGF-β as secreted cytokines (Castro-Sánchez and Martín-Villa, 2013). Although these cytokines have different functions, they work together to regain the original state of the body (Roncarolo et al., 2006; Castro-Sánchez and Martn-Villa, 2013). Studies have reported the potential of certain probiotic LAB to downregulate proinflammatory cytokines such as IL-1, IL-6, and TNF-α while upregulating the production of anti-inflammatory cytokines (IL-10 and TGF-β) (de Moreno et al., 2011). In this study, L. fermentum NBRC 15885 demonstrated the highest levels of serum IL-10 and TGF-β, whereas L. fermentum CIP 102980 and L. plantarum CIP 103151 also demonstrated similar results. L. pentosus 124-2 only upregulated the TGF-β production with no significant impact on IL-10 production. The decrease in the blood levels of the proinflammatory mediator CR-P in the treated rat groups may be because IL-10 has been shown to limit the generation of proinflammatory cytokines. The paw size of the positive control (i.e., a standard NSAID diclofenac sodium) reduced significantly; however, the MPO activity showed that diclofenac sodium had no significant effect on neutrophil infiltration, failing to control its influx, whereas all LAB isolates, especially L. fermentum NBRC 15885 and L. plantarum CIP 103151, showed a tremendous early effect on neutrophil accumulation in the paw of rats. This proves that the LAB species in this study must have used a different pathway than the known mechanism used by NSAIDs, as described by Amdekar et al. (2012), which involved the regulation of the synthesis of the recognized prostaglandin-producing enzyme coxygenase-2. This suggests that LAB have a lower probability of causing gastrointestinal toxicity, which results from the aforementioned pathway, which is significant. It is also possible to hypothesize that cytokine pathways are a key mechanism used by LAB in the control of acute inflammation because most immune cells frequently respond to immune signaling by cytokines, and the present results revealed that CR-P (a pro-inflammatory cytokine) was controlled and anti-inflammatory cytokines (IL-10 and TGF-β) were upregulated in LAB treatments. These results demonstrate that this anti-inflammatory cytokine production response occurred at the early hours (8 and 24) of inflammatory responses, which confirms that the activity of LAB triggers CD4+ T regulatory cells probably via interaction with the mucosal and/or intestinal epithelial cells, which eventually results in earlier resolution of inflammation (Wells, 2011). The findings of the present study imply that anti-inflammatory cytokines play an active role in the mechanism of LAB species to resolve acute inflammatory responses; however, further studies are required to determine the specific mechanisms and pathways involved in this process.
Conclusions
By increasing anti-inflammatory cytokines and modulating neutrophil infiltration to the paw of rats administered carrageenan injections, LAB prevalent in fermented food products used in this study showed immunomodulatory potential. This suggests that the function of LAB species in the resolution of acute inflammatory responses is actively influenced by anti-inflammatory cytokines. In the absence of NSAIDs, Nunu and Ogi can be used as a conventional and alternative form of treatment for inflammation.